- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Bioremediation of Agro-Wastewater of Poultry in a Microbial Fuel Cell
Livinus A Obasi1* and Okechukwu D Onukwuli2
1Department of Chemical Engineering, Federal Polytechnic Ekowe, Nigeria
2Department of Chemical Engineering, Nigeria
*Corresponding author:Livinus A Obasi, Department of Chemical Engineering, Federal Polytechnic Ekowe, Bayelsa state, Nigeria
Submission: July 29, 2019;Published: August 22, 2019

Volume2 Issue2August, 2019
Abstract
The study shows the possibility of degrading pollution physico-chemical parameters present in agro wastewater (poultry) in a microbial fuel cell with microorganisms operating in an anaerobic condition. Three similar cells designed and operated with different Proton Exchange Membranes (PEM) makeups: clay-based (CBP) whose strength and stability were modified with sodium alginate (C6NaH7O6) n and subjected to varying degrees of temperatures ranging from 200 °C to 600 °C and applied in (MFC-1, MFC-2 and MFC-3. The cells were operated for 20 days at influent BOD and COD of 493mg/l and 2052mg/l respectively. The study showed a reduction in the turbidity, pH, Total Dissolved Solid (TDS), Total Suspended Solid (TSS) and salinity of the wastewater. After twenty days of operation, the BOD removal efficiencies of the three reactors were 72%, 82% and 84% respectively, whereas the COD removal efficiencies were 38%, 57% and 60% respectively. The study also shows that the MFC-3 at 6000C PEM preparation condition gave the optimum performance giving the lowest values of the various wastewater pollution parameters. This clearly suggests that within the conditions of the experimentation process, clay performs optimally in proton transfer from the anode chamber to the cathode chamber of a microbial fuel cell at higher temperatures due to increase in cation exchange capacity.
Keywords: Microbial fuel cell; BOD; Poultry wastewater; Clay-PEM; Bioremediation
Introduction
The need to achieve effective control of environmental pollution due to discharge of liquid waste at a relatively low cost lies the advantage of deploying microbial fuel cell technology. The operation of this device is essentially a departure from the conventional aerobic treatment which has a higher operational cost implication, to the use of anaerobic method [1].
Besides, the search for alternative sources of energy has increased due to current prediction of the global energy depletion [2]. There is therefore a clarion call for sustenance of the worldwide energy demand and a balance of the effect of energy demand chain operation on the environment. The challenge, therefore, is that fossil fuel as the hub of world energy supply is exhaustible implying that several centralized power plants probably may become redundant due to lack of fuel. Hence the focus is now on the quest for strategic solution designed this growing world energy demand [3].
Research has so far shown that microbial fuel cell is able to generate electricity via anaerobic microbial degradation of organic substance present in wastewater with concomitant effect of treating the wastewater [4]. The components of the cell are basically the reactors holding the wastewater (anode chamber) and the electron sink (cathode chamber). The Proton Exchange Membrane (PEM) constitutes a salt bridge connecting the two chambers through which protons are transferred from the anode chamber to the cathode chamber [5]. The efficiency of the PEM is a measure of its ability to achieve high percentage of the proton transfer under the given experimental conditions. However, at lower proton transfer rate, the tendency for interaction of the released proton with the dissolved and suspended particles in the wastewater increases tremendously. This destabilizes the suspended particles via neutralization of the surface charges to induce flocculation and sedimentation.
The current status of world energy put dependence on fossil fuel at 80±5% while renewable and nuclear energy completes the balance. The resultant effect of this is that many countries are in short supply of energy due to cost and technology. It is also important to note that transportation alone gulps 58% of the world fossil fuel supply [3,6,7].
The handling of anthropogenic waste in an environmentally friendly manner gives tremendous challenges to researchers, inventors, engineers, scientists, policy makers and economist alike [8,9]. More so these materials may be made into a resource to support energy supply type like the off grid as they are not feed stocks having competitive demand, but they can be turned into valuable energy resources [10]. Thus, the worries caused by fast depleting fossil fuel have heightened the search for high and efficient energy transformations with leakage on the utilization of renewable energy sources as a leeway [7]. Therefore, a major shift in energy sources for more efficient renewable energy sources is desirable due to a gradual obsolescence of fossil fuels including coal and petroleum [11].
Materials and Methods
Preparation of electrode
The electrode used in this study was the locally manufactured type whose base material was charcoal from saw dust obtained from a sawmill located around university of Port Harcourt, Choba, Nigeria. About 20g of pulverized charcoal was mixed with 5g of cement and the mixture was subjected to a temperature of about 400 °C in a muffle furnace for one hour. The critical function of this component was to enhance the transfer of electrons generated via microbial activity in the anode chamber during cell operation to the external circuit. The efficiency and performance of each cell depends largely of the biomass base and the temperature at which each electrode was prepared.
Preparation of proton exchange membrane (PEM)
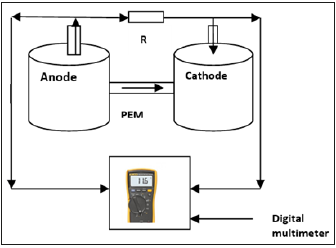
The microbial fuel cell used was the dual chambered H-type (Figure 1). A PVC pipe of internal diameter 0.003m and length 0.08m was fitted firmly between the two MFC reactors while epoxy adhesive polymeric substance was used to seal the contact point between the reactors and the pipe housing the proton exchange membrane. The pipes were sterilized by cleaning and disinfecting the internal surface with alcohol to preempt the possible interfering activity of microorganisms prior to cell operation.
Clay-based proton exchange membrane (CB-PEM)
The medium of proton exchange was basically clay whose strength was improved with the addition of sodium alginate polymeric substance. Clay is type of soil (commonly composed of phyllosilicate mineral (Si2O5)2-) especially the muscovite white mica (KAl2(AlSi3O10) (OH)2 with fine grain particles that are closely packed together to give it a non-porous nature. It possesses characteristic high cation exchange capacity [12-15]. This mineral was first thermally treated by subjecting it to autoclave temperature of 600 °C for about 30 minutes. Thereafter about 5g of sodium alginate (C6H7NaO6)n (IUPAC-Sodium;3,4,5,6-tetrahydroxyoxane- 2-carboxylate) yellowish granular polymeric substance softened with hot water was added in order to enhance the release of cation exchange sites.
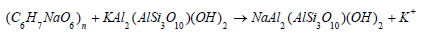
Anolyte (substrate), Inoculation and Start-up
The feed stock to the anode chamber (poultry wastewater from poultry dropping ratio of 3:1(w/w) was properly mixed and allowed to settle out the non-soluble matter which was separated from the supernatant by decantation. Three aliquot samples of the supernatant of each effluent sample were prepared in the following ways. A 5ml bacteria (mixed consortium environmental inoculum) laden soil solution was added to each of the and fed into each of the three cells and labelled samples A1, A2 and A3 (Table 1).
Table 1:Physiochemical result of poultry wastewater samples.
The enrichment of sample A3 of the supernatant with 10% glucose solution is aimed at establishing the effect of increased growth and energy of microbe metabolic activity while generating proton and electron.
Catholyte Preparation
The catholyte used in the study were of two sorts; one was prepared with 0.1M potassium ferricyanide (0.1M k3Fe(CN)6 solution buffered with potassium dihydrogen hotophosphate (0.1MKH2PO4) solution to keep the operating pH at 7.5, while the second catholyte was prepared with produce water (pH 7.1) and fully aerated. The charcoal-cement electrode (cathode) was fixed and suspended in the catholyte to provide the means of electron transfer into the catholyte for reduction reaction (electron sink).
Result and Discussion
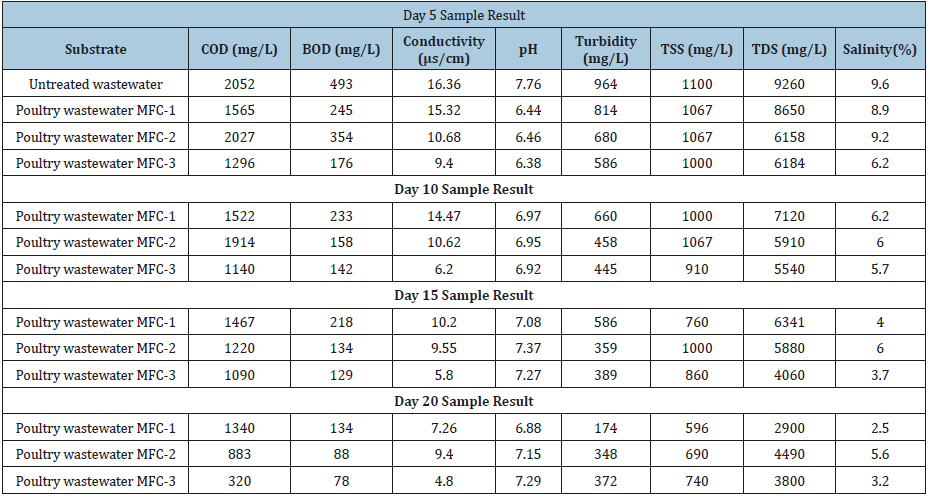
In this experimental study, effort was made to determine the extent to which the number of various parameters that characterize poultry wastewater samples as feedstock in a microbial fuel cell was degraded during cell operation. It was observed that, the biological activity of bacteria in the wastewater, in addition to producing electrons, degrade the pollution parameters such as BOD, COD, and further cause the destabilization and coagulation of suspended and dissolved particles in the wastewater. The values of these parameters were found to decrease with time (day) and also with increase in the temperature of treatment of clay used as the Proton Exchange Membrane (PEM), and also with the operating pH, electrolyte concentration and electrochemical time. In order to optimize the potential of MFC in water and wastewater treatment, Electrocoagulation Process (EC) was investigated by determining the level of solid particles suspended or dissolved in the electrolyte. These particles were able to settle as flocs due to the destabilization and flocculation of the negatively charged particle via interaction with the generated protons from the activity of the microorganisms in the anode chamber. The initial pH, turbidity, TSS, TDS at time t=0 were 7.76, 964,1100 and 9260mg/L respectively and were reduced to 6.88, 174, 596 and 2900mg/L after 20 days of cell operation.
Figure 1:A schematic diagram of MFC reactors with clay-based PEM
The data obtained from each cell during operation are as shown in the Appendix. Figures 1-6 are the graphical representations of the performance of each cell with respect to the biodegradation of the pollution parameters as shown below.
Figure 2:Profile of COD and BOD of poultry wastewater against time (Days).
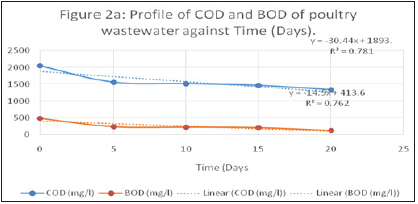
Figure 3:Profile of conductivity and pH of poultry wastewater against Time (Days).
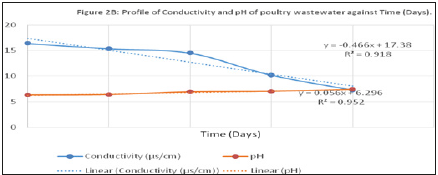
Figure 4:Profile of turbidity og poultry wastewater (mg/l) against Time (Days).
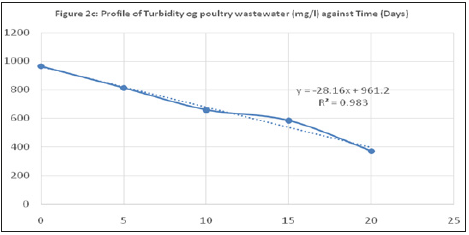
Figure 5:Profile of TSS and TDS of poultry wastewater against Time (Days).
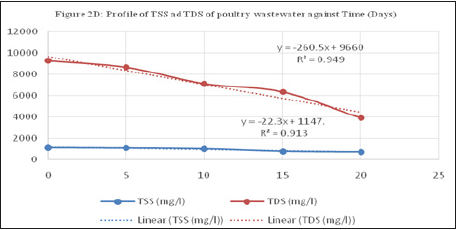
Figure 6:Profile of voltage and current against time (days) for MFC-1 at 200 deg cent.
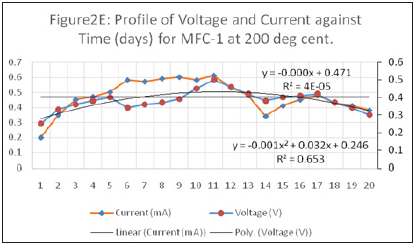
A simplified mathematical model for MFC was developed to predict the current-voltage-power generation relationship with time and the decay constant for the batch operated MFC.
Decay constant
The evaluation for the first-order kinetic of the oxygen consumption, the changes in the oxygen available relates to the BOD which also establishes the decay rates for water quality models [16,17]. The model for the kinetic shows BODt correlation
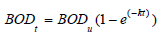
The decay constant of the different electrodes was determined to be 6.039x10-3s-1, 1.039x10-2 s-1 and 9.74x10-3 s-1 for reactors operated with MFC-1, MFC-2 and MFC-3 respectively.
The anode chamber reactions with respect to electron transfer (redox reactions) in a poultry wastewater are as follow:
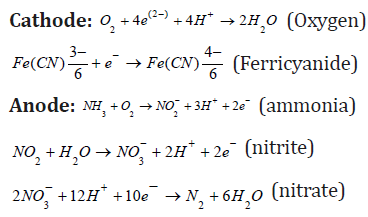
BOD and COD removal efficiencies
The physicochemical parameters of the wastewater were determined for each of the three MFC reactors operating with different electrode materials and PEM at different temperatures (Reactor Tag: MFC-1, MFC-2, MFC-3), operated at influent BOD and COD of 493mg/l and 2052mg/l respectively. After a 20-day cell operation with the cell parameter values recorded at 5 days interval, the removal efficiency of the reactors was 72%, 82% and 84% for the MFC-1, MFC-2 and MFC-3 reactor respectively.
The COD removal efficiency of the three reactors using the different electrode materials and Proton Exchange Membranes (PEM) were 38%, 57% and 60% for the MFC-1, MFC-2, and MFC-3 respectively. The behavior of the BOD and COD as observed in terms of their removal efficiencies.
These observations are lower compared with the findings of Ghangrekar & Shinde [4] who reported 90% COD and BOD removal with synthetic wastewater operated for 55 days and but higher than the findings of Min & Logan [14] that achieved 42% removal with domestic wastewater and organic substrates influent. While the findings are within the COD removal efficiency of 20 to 80% reported by Aelterman et al. [13] from a study using wastewater from a hospital.
Poultry wastewater conductivity and pH treatment
Conductivity of water is a measure of its ability to conduct electricity. This tendency is directly related to the number of conductive ions present in the water which could come from either dissolved salts or inorganic materials. Conductivity (measured in micro siemens- or milli siemens per centimeter) (μs/cm) varies linearly with the number of ions present in the water. The conductivity of the influent wastewater (feed stock) was 16.36μs/ cm. In the course of the operation of the cells, conductivity of the three reactors reduced gradually to a minimum value after 20 days to 7.26, 9.4 and 4.8μs/cm for MFC-1, MFC-2 and MFC-3 respectively. This downward trend could have been as a result of the decrease in the ions in the electrolyte due to increased rate of oxidation and transfer of particles.
pH is a logarithmic function of the amount of hydrogen ions in a substance or solution. The pH of influent wastewater fed into the reactor was 7.76. The pH recorded at the end of the study period were 6.88, 7.15 and 7.29 for the three cells respectively. The reduction in the operating pH could have been as a result of the increase in the influx of hydrogen ions (protons) present in the anode chamber. The cell whose clay-based proton exchange membrane (CBP) was prepared at 600 °C gave the least (6.88). This suggests that there was reduced rate of transfer of proton through a membrane at prepared at low temperature than could be obtained at higher temperatures. This may be related to the possibility of CO2 production at the anode chamber due to anaerobic oxidation (metabolic) process giving rise to formation of a weak acid as shown in equations 2 to 5 below. These results tend to agree with the finding that there is significant influence of divalent compounds bulk substrate on MFC performance suggested by Argun et al., (2007).
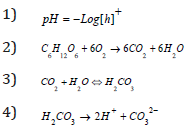
Poultry wastewater turbidity and TSS treatment
Turbidity is a measure of the cloudy or murky nature of water as a result of presence of dissolved or suspended particles (fine organic and inorganic, colored organic compounds, microorganisms) capable of scattering light. Turbidity is measured in Nephelometric Turbidity Unit (NTU).
The influent wastewater turbidity reduced for the various reactors from the influent value of 964 NTU to 374, 298 and 262 NTU for MFC-1, MFC-2 and MFC-3 respectively. Also, the total suspended solid, TSS of the influent wastewater reduced from feed value of 1100mg/L for the feed to a minimum value of 696, 690 and 740mg/L for the three MFCs respectively with removal percentages of 36.72, 37.27 and 32.72%. this reduction could be as a result of electrocoagulation process caused by the interaction of the released protons from microbial activity with the suspended particles, neutralized their negative charges and caused them to aggregate and settle as sludge. This TSS reduction is however low as compared to that reported by Min et al. [14], pH adjusted MFC reactor.
Poultry wastewater TDS and salinity treatment
The total dissolved solid in the poultry wastewater solution reduced from the first day until the 20th day of the experiment. From the initial value of 9260mg/l, the MFC-1 had the highest reduction in TDS to 3800mg/l followed by the MFC-2 with reduction in TDS to 2900mg/l.
On the treatment of the salinity of the wastewater, the best performance occurred using the PEM prepared at 200 °C reducing the value from 9.6% to 2.5 [15,16].
Conclusion
The study established the potentiality of the device for biotreatment of poultry wastewater. The MFC deployed in this research is a dual chamber salt bridge H-type. The anode electrolyte made of poultry waste solution and the cathode chamber of potassium ferricyanide solution with charcoal-cement composite as electrode. The clay-based proton exchange membrane was prepared at different temperatures ranging from 200 °C to 600 °C. The cell with 200 °C PEM preparation temperature gave the best performance in reducing the wastewater pollution parameters. This was linked to the fact that less proton transfer rate gave way for sufficient proton needed for interacting with the suspended and dissolved solids to induce coagulation and sedimentation. The level of treatment of the wastewater was monitored using Physico-chemical parameters in the raw and treated water. The parameters measured include the chemical oxygen demand, biochemical oxygen demand, pH, conductivity, turbidity, Total Suspended Solid (TSS), Total Dissolved Solid (TDS) and salinity [17,18].
The Chemical Oxygen Demand (COD) was reduced from 2052mg/L to 134mg/L, Biochemical Oxygen Demand (BOD) from 493mg/L to 134mg/L, pH 6.68, conductivity 16.36μs/cm to 7.26μs/ cm , turbidity 964 to 174 NTU, Total Suspended Solid (TSS), 1100 to 596mg/L, Total Dissolved Solid (TSS) 9260mg/l to 2900mg/L and salinity 9.6 to 3.8%. The experimental laboratory study has shown that MFC with poultry wastewater as feedstock can generate current which can be scaled up for poultry farm use.
References
- Aelterman P (2009) Microbial fuel cells for the treatment of waste streams with energy recovery. PhD Thesis, Gent University, Belgium.
- Haslett ND (2012) Development of a eukaryotic microbial fuel cell using Arxula adeninivorans. PhD thesis, Lincoln University, Chirstchurch, New Zealand.
- Sørensen B (2004) Renewable energy: Its physics, engineering, use, environmental impacts, economy and planning aspects. (3rd edn), Elsevier Science, New York, USA, pp. 29-360.
- Ghangrekar MM, Shinde VB (2008) Simultaneous sewage treatment and electricity generation in membrane-less microbial fuel cell Water Sci Technol 58(1): 37-43.
- Obasi LA, Opara CC, Oji A, (2012) Performance of cassava starch as a proton exchange membrane in a single dual chamber microbial fuel cell. International Journal of Engineering Science and Technology (IJEST) 4(1): 227-238.
- Nigam PS, Singh A (2011) Production of liquid biofuels from renewable resources. Progress in Energy and Combustion Science 37(1): 52-68.
- Kuye AO, Edeh I (2013) Production of bio-oil from biomass using fast pyrolysis: A critical review. Journal of Minerals Research 1(1): 1-20.
- Pham TH, Rabaey K, Aelterman P, Clauwaert P, Schamphelaire, et al. (2006) Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng Life Sci 6(3): 285-292.
- Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/Biomass for Bio-oil: A critical review. Energy & Fuels 20(3): 848-889.
- Cheng S, Kiely P, Logan BE (2011) Pre-acclimation of a wastewater inoculum to cellulose in an aqueous–cathode MEC improves power generation in air–cathode MFCs. Bioresour Technol 102(1): 367-371.
- Rosenbaum M (2007) Biofuels: Microbial fuel cells: Making waste into power. Washington University in St. Louis, Science Outreach, USA.
- Emeniru DC, Onukwuli OD, Wodu PD, Momohjimo AO (2015) Adsorption characteristics of ekowe clay and uptake kinetics of methylene blue onto the raw and modified clay. International Journal of Multidisciplinary Sciences and Engineering 6(5): 9-19.
- Aelterman P, Rabaye K, Boon N, Verstraete W (2006) Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ Sci Technol 40(10): 3388-3394.
- Min B, Logan BE (2004) Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ Sci Technol 38(21): 5809-5814.
- Argun ME, Dursun S, Ozdemir C, Karatas M (2007) Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. Journal of Hazardous Materials 141(1): 77-85.
- American Public Health Association (2005) Standard methods for the examination of water and wastewater. (21st edn), Washington DC, USA.
- Sullivan AB, Snyder DM, Rounds SA (2010) Controls on biochemical oxygen demand in the upper Klamath River, Oregon. Chemical Geology 269(1-2): 12-21.
- Aelterman P, Rabaey K, Clauwaert P, Verstraete W (2006) Microbial fuel cells for wastewater treatment. Water Science & Technology 54(8): 9-15.
© 2019 Stancu Mihaela Marilena. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






