- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Study on Plant Growth Promoting Activities of Azotobacter Isolates for Sustainable Agriculture in Myanmar
Phyu KE*
Department of Biotechnology Research, Myanmar
*Corresponding author:Department of Biotechnology Research, Myanmar
Submission: April 04, 2019;Published: April 23, 2019

Volume1 Issue5April, 2019
Abstract
Plant growth promoting Rhizobacteria (PGPR) enhance the plant growth and productivity through wide variety of mechanism. In this paper, a total of 6 isolates belonging to Azotobacter spp. were isolated from different rhizosphere soils collected from Kyaukse Township, Mandalay Region. The isolated bacterial cultures were characterized morphologically, culturally and biochemically and then they were identified as Azotobacter. They were screened for their plant growth promoting traits. Results revealed that isolated strains are capable of fixing nitrogen, solubilizing phosphorus and synthesizing indole compounds. It was concluded that among the isolates, “U” is the best isolate for all these activities. The results of the present experiments can be utilized in biofertilizer production to provide sustainability to the agricultural productivity.
Introduction
Myanmar is an agricultural country and out of 67.6 million hectares of land in Myanmar, 12.8 million hectares are cultivated land. When measured by value of production, rice is the dominant commodity, accounting for 43% of production value. Agriculture is the backbone of the Myanmar economy: The sector accounts for about 30% of GDP, over 50% of total employment and approximately 20% of exports [1]. Economy of our country is greatly dependable on agriculture. The more the agriculture develops, the higher the standards of our country. Therefore, the demand for fertilizer is very high. Sustainable agricultural production requires new approaches to reduce the applications of polluting agrochemicals [2]. One potential solution to overcome this major challenge is the study of the biological processes involving plant growth-promoting rhizobacteria (PGPR/PGPB) and their interaction with plants [3].
Plant growth promoting rhizobacteria (PGPR) are soil borne bacteria, which enhance the plant growth directly or indirectly. PGPR can exhibit a variety of characteristics responsible for influencing plant growth [4]. Among the different bacterial genera that have been reported as PGPR (Azospirillum, Agrobacterium, Rhizobium, Enterobacter, Beijerinckia, Klebsiella, Xanthomonas, Phyllobacterium) [5], Pseudomonas, Bacillus and Azotobacter are the most widely reported [6-9]. Several lines of evidence suggesting direct mechanisms of PGPR/PGPB involved in plant growth promotion are the following
A. Production of ACC deaminase, which reduces the level of ethylene in crop roots thus enhancing root length and density.
B. Symbiotic and associative nitrogen fixation, which increases the availability of soluble nitrogen in soil.
C. Nutrient solubilization and mineralization (e.g. P, K, Zn and Si), which increases the availability of those elements for plant uptake.
D. Synthesis of phytohormones such as indoles, gibberellins, abscisic acid and cytokinin’s which modulate plant growth and division.
E. Ability to produce siderophores, hydrolytic enzymes and antibiotics, which renders the cells more competitive over niche colonization.
F. Quorum sensing signal interference and inhibition of biofilm formation.
G. Enhance resistance to stresses by synthesis of watersoluble vitamins as niacin, thiamine and biotin 4,12 [10,11].
Within the PGPR/PGPB group, the nitrogen-fixing bacterium Azotobacter chroococcum has shown to promote the growth of different crops under different soil types and climatic conditions [12]. Among the heterotrophic free-living N2-fixing bacteria, Azotobacter is the most intensively investigated genera. Apart from its ability to fix atmospheric N, Azotobacter is also known to synthesize biologically active growth-promoting substances such as indole acetic acid, gibberellins and B-vitamins in culture media [13]. Azotobacter is free living bacteria and is widely distributed in different types of soils. Azotobacter are present in neutral or alkaline soils and A. chroococcum is the most commonly occurring species in arable soils.
Azotobacter is a nitrogen fixer so it enhances the plant development, produces plant growth regulators and increases mineral phosphates solubility by producing hydrogen cyanide, siderophore. Azotobacter has antifungal activity it produces antibiotics [14]. Azotobacter has been used as a potential nitrogenous fertilizer to increase crop growth. The Azotobacter genus was discovered in 1901 by Dutch microbiologist and botanist Beijerinck (founder of environmental microbiology). A chroococcum is the first aerobic free-living nitrogen fixer. They multiply rapidly and develop a thick population in rhizosphere, when applied as seed treatment or seedling root-dip or as soil application [15]. They are non-symbiotic heterotrophic bacteria capable of fixing an average 20kg N/ha/year under optimum conditions and increase yield up to 50%.
Azotobacter species can tolerate and survive in extreme environmental condition by producing cysts [16]. The occurrence of this organism has been reported from the rhizosphere of a number of crop plants such as rice (Oryza sativa L.), maize (Zea mays L.), sugarcane (Saccharum officinarum L.), bajra (Pennisetum glaucum L.), vegetables and plantation crops [17]. The main purpose of this research was to study the plant growth promoting efficiency of rhizobacteria (PGPR)-Azotobacter.
Material and Methods
Collection of soil samples
Soil samples were collected from the rhizosphere region of five different paddy fields of Kyaukse Township, Mandalay Region, Myanmar. Samples were collected in polythene bags from the selected sites at a depth of 10-15cm. The samples were then immediately transported to lab for further processing.
Isolation of Azotobacter species from paddy fields
To cultivate bacteria from soil samples, one gram of soil was placed in the sterile test and 5ml of 0.9% NaCl was added to it. Vigorous shaking of the tube was made to dissolve those soluble components. The tube with soil samples were kept standing for about 10 minutes, so that heavy materials become settled down. Then 1ml of upper portion of the soil suspension was drained and precipitate was spread on Jensen’s medium that was used as selective medium for Azotobacter. Plates were then incubated at 30 °C and examined after 7 days for growth.
The colonies that developed were picked based on morphological characteristics. Each colony picked was subcultured into the respective enrichment media using a sterile wire loop under aseptic conditions to streak onto the duplicate plates and incubated at 30 °C for 24-48 hours. After incubation, the cultures were sub-cultured to get discrete colony. The colonies that developed were then Gram-stained. The discrete colonies produced from previous subculture was picked with the aid of a sterile wireloop and inoculated onto 10ml of peptone water and incubated for 24hrs at 30 °C before being stored properly in the refrigerator. This served as the stock culture.
Morphological and biochemical characterization
The phenotypic characteristics of isolated strain were studied and characterized such as colonial and microscopic morphology, some biochemical characteristics by using the criteria described in Bergey’s Manual of Systematic Bacteriology [18,19].
Characterization for plant growth promoting traits
Assay for IAA production: The production of indole acetic acid (IAA) by selected 6 strains of nitrogen fixing bacteria and the effect of L-tryptophan on IAA production was assayed by using a colourimetric technique and it was performed with Van Urk Salkowski reagent using the Salkowski’s method [20]. The isolates were inoculated in the respective medium with tryptophan containing 0.5g/l of tryptophan and incubated at 30 °C for 14 days. The broth was centrifuged after incubation. Supernatant was reserved and 1ml was mixed with 2ml of Salkowski’s reagent (2% 0.5M FeCl3 in 35% perchloric acid solution) and kept in the dark. The optical density (OD) was recorded at 530nm after 30min. The level of IAA produced was estimated by standard IAA graph.
Qualitative determination of phosphate solubilizing activity
To determine the solubilization of phosphate, National Botanical Research Institute Phosphate (NBRIP) media and Pikovskaia’s media were used and the single colony of bacterial strains was spotted on NBRIP media and Pikovskaia’s containing BTB that it was supplemented with tricalcium phosphate as substrate. After five days of incubation, the colonies showing the yellow halo zone around them were considered as positive and halo zone diameter were recorded.
Quantitative determination of phosphate solubilizing activity
For the quantitative estimation of soluble phosphates, the bacterial strains were inoculated in Pikovaskaia’s broth media in a flask (10ml) and incubated in water bath shaker at 30 °C for five days. Uninoculated medium served as control. After incubation, the culture broth was passed through the cation exchange resin and (PO4) 3-solution was reacted with colour forming reagent (Sodium Molybdate and Hydrazium Sulphate). After blue colour development, phosphate solubilizing activity was measured by UVvis spectrophotometric method at 830nm [21].
Qualitative determination of nitrogen-fixing activity
The visual detection of nitrogen-fixing activity was observed by using glucose nitrogen free mineral agar medium containing BTB (Bromothymol blue solution). After one-week incubation, changing the colour of BTB containing medium was recorded [22].
Quantitative determination of nitrogen-fixing activity
The isolated strains were screened for nitrogen fixingactivity by using glucose nitrogen free mineral agar medium as well as broth medium and ammonia test kit (VISCOLOUR alpha ammonium reagent, MACHEREY-NAGEL GmbH & Co. KG, Germany). The pure cultures were inoculated into GNFMM containing BTB (bromothymol blue solution) and without BTB and incubated in a shaker for a week and changing the colour of medium from green to blue was recorded.
For the determination of nitrogen fixing activity from NFGMM broth medium without BTB, broth culture was centrifuged at 8000rpm for 10 minutes and the pellet was discarded. Two drops of ammonium test kit Reagent I was mixed with 1ml of supernatant. And one fifth of Reagent II was added and incubated for five minutes. After that, one drop of Reagent III was poured and incubated again for 5 minutes. Finally, colour development was noted by comparing the colour chart from the test kit [22].
Result and Discussion
Isolation of Azotobacter sp. from paddy fields
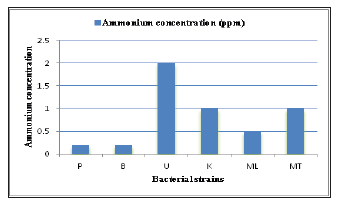
Six bacterial strains were isolated from different paddy soils on Jensen’s N-free medium at 30 °C for 24hrs incubation. Gram staining, motility and biochemical tests such as indole, methyl red, vogesproskauer, citrate, urease, nitrate, catalase, oxidase, utilization of different carbon sources, starch hydrolysis, gelatine hydrolysis was performed. Bacteria were Gram-negative with rounded ends, with an average cell size of 1.3×4.0μm after 24h growth. They occurred singly, in paired or irregular clumps and sometime in chains of varying length. They cannot produce endospores but form cysts that can tolerate and service in extreme environmental conditions and Salhia said that it is means of asexual reproduction under favorable condition [23].
The biochemical test was performed as catalase activity test. The test shows the ability of some microbes to degrade hydrogen peroxide by producing the enzyme catalase. Usually, all Azotobacter species have the capacity to produce oxidases and catalases for the protection of their nitrogenase [24]. The colonies were glistening; smooth, slimy, mucoid, semi-transparent during the early growth and later brown on Jensen’s N-free agar plates. In my research work, the colonies of isolated strains produced brown pigment after 7 days of incubation. According to literature, pigments are also an important characteristic and produced by all Azotobacter spp. For example, A. chroococcum forms a dark-brown water-soluble pigment melanin.
This process occurs at high levels of metabolism during the fixation of nitrogen and is thought to protect the nitrogenase system from oxygen [25]. Other Azotobacter species produce pigments from yellow-green to purple colours [26] including a green pigment which fluoresces with a yellow-green light and a pigment with bluewhite fluorescence. So, it can be assumed that all the isolates may be Azotobacter chrocioccum. The morphological and biochemical characteristics of the isolated strains were compared with those defined in manual [19] to confirm them as Azotobacter strains (Table 1).
Table 1:Biochemical characteristics of bacterial isolates strains.
Production of indole-3-acetic acid
PGPR can exhibit to produce plant growth regulators (auxins, gibberellins, and ethylene). Indole acetic acid is a common product of L-tryptophan metabolism by several microorganisms including PGPR [27]. All isolated strains in a culture medium containing tryptophan of 0.5g/l produced IAA as detected by the Salkowski reagent under colourimetry in the range of 20mg/l to 90mg/l Figure 1. “MT” strain produced the highest amount of IAA concentration. Figure 2 showed the samples of the obtained solutions of this compound. Many bacteria isolated from the rhizosphere have the capacity to synthesize IAA in vitro in the presence or absence of physiological precursors, mainly tryptophan (Trp) [28]. It has been reported that IAA production by PGPR can vary among different species and it is also influenced by culture condition, growth stage and substrate ability [29,30] (Figure 1 & 2).
Figure 1:Detection of IAA concentration of isolated bacterial strains by UV vis spectrophotometer at 530nm.

Figure 2:Screening of IAA producing activities of isolated strains by Salkowski reagent.

Phosphate solubilizing activity
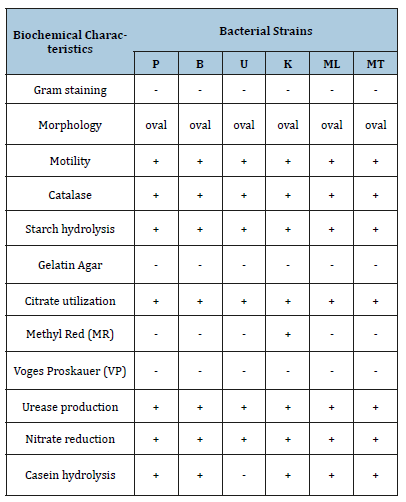
Large proportion of phosphorus in soil is insoluble and therefore unavailable to plants [31]. The ability of some microorganisms to convert insoluble Phosphorus (P) to an accessible form, like orthophosphate, is an important trait in a PGPB for increasing plant yields [32]. In this research, all the selected isolates were found to be potent phosphate solubilizers showing yellow halo zone around the colonies on NBRIP media and Pikovskaia’s media Figure 3. In this screening method, they gave higher halo zone diameter in NBRIP media than in Pikovskaia’s media Figure 4.
After plate screening, phosphate solubilizing activity of all selected strains were quantitatively determined by UV-vis spectrophotometric method at 830nm using KH2PO4 as standard. In qualitative analysis of phosphate solubilizing activity, “U” strain gave the highest amount of phosphate solubilization Figure 5. Some researchers noted that certain enzymes (e.g. acid phosphatases) could play a major role in the mineralization of organic P in soil [33] (Figures 3-5).
Figure 3:Screening of phosphate solubilizing activity of isolated strains by yellow halo zone formation on Pikovskaia’s and NBRIP media with BTB.

Figure 4:Screening of P-solubilizing activities of NBRIP and Pikovskaia’s media with BTB by halo zone diameter.
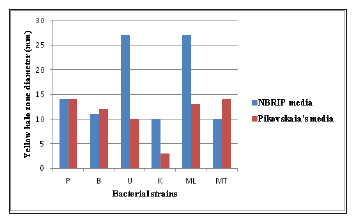
Figure 5:Detection of P-solubilizing activity of isolated strains by UV vis Spectrophometric method.
Nitrogen fixing activity of isolated strains
For screening of nitrogen fixation activity, all bacterial strains were incubated in G-NFMM solid media containing BTB and incubated for one week. After one-week incubation, the best isolates for nitrogen fixation produced significant amounts of ammonia into the media by changing the colour of the medium from green to blue as the pH of the medium was increased Figure 6. Plate screening method was used using BTB as indicator. Before culturing on G-NFMM, the colour of media was green. But, after culturing of G-NFMM for one week, the colour of the media was turned from green to blue.
Figure 6:Screening of nitrogen fixing activities of isolated strains on G-NFMM with BTB.

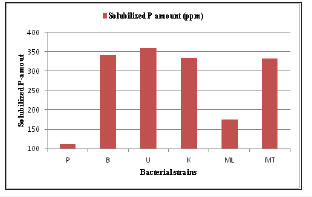
Control media without the bacteria remained as green colour. It was assumed that NFB accumulated excreted ammonia into media and pH of the media was increased [22]. Consequently, this reaction caused the changing of the colour of the media. For quantitative analysis for nitrogen fixing activity, all isolated strains gave nitrogen fixing activity when activity was tested by ammonium test kit. “U” strain showed higher amount of ammonia than other five strains in detection with ammonium test kit (Figures 6-8).
Figure 7:Nitrogen fixing activities of isolated strains by ammonium test kits

Figure 8:Detection of nitrogen fixing activity of isolated strains by ammonium test kit.
Conclusion
Azotobacter could be one of the bio-fertilizer options for sustainable and environmental eco-friendly. This study confirmed that Azotobacter have the plant growth promoting activities (nitrogen fixing activity, phosphate solubilizing activity and indole acetic acid producing activity). In quantitative analysis for these activities, all Azotobacter isolates gave the considerable amount of for IAA, nitrogen fixation and phosphate solubilization and “U” strain is the best strain for all these activities. This isolated U strain may be further enhanced with the optimization and acclimatization according to the prevailing soil conditions.
In future, they are expected to replace the chemical fertilizers, pesticides and artificial growth regulators which have numerous side-effects to sustainable agriculture. Azotobacters are unique biofertilizers to maintain the N level in agriculture soil. Coinoculants increased most of growth parameters, water and nutrient uptake under the deficit irrigation. Azotobacter has a potential to stress tolerance that results crop improvement. Hence, understanding and manipulating this feature may be of great agroecological interest for future crop improvement.
References
- Bank Asian Development (2013) Myanmar: Agriculture, natural resources, and environment initial sector assessment, strategy, and road map. Asian Development Bank, Philippines, USA.
- Kraiser T, Gras DE, AG Gutiérrez, González B, Gutiérrez RA (2011) A holistic view of nitrogen acquisition in plants. Journal of Experimental Botany 62(4): 1455-1466.
- Adesemoye AO, Kloepper JW (2009) Plant-Microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol 85(1): 1-12.
- Kloepper JW, John L, Martin T, Milton NS (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286: 885-886.
- Lucy M, Reed E, Glick BR (2004) Applications of free-living plant growthpromoting rhizobacteria. Antonie van Leeuwenhoek 86(1): 1-25.
- Mrkovački N, Bjelić D (2011) Plant Growth Promoting Rhizobacteria (PGPR) and their effect on maize. Ratarstvo I Povrtarstvo 48(2): 305- 312.
- Joo GJ, Kim YM, Lee IJ, Song KS, Rhee IK (2004) Growth promotion of red pepper plug seedlings and the production of gibberellins by bacillus cereus, bacillus macrolides and bacillus pumilus. Biotechnol Lett 26 (6): 487-491.
- Poonguzhali S, Munusamy M, Tongmin S (2008) Isolation and identification of phosphate solubilizing bacteria from Chinese cabbage and their effect on growth and phosphorus utilization of plants. J Microbiol Biotechnol 18(4): 773-777.
- Đurić S, Pavić A, Jarak M, Pavlović S, Starović M (2011) Selection of indigenous fluorescent pseudomonad isolates from maize rhizosphere soil in Vojvodina as possible PGPR. Romanian Biotechnological Letters 16(5): 6580-6590.
- Bernard RG (2012) Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, Article ID 963401, pp. 1-15.
- Bhattacharyya PN, Jha DK (2012) Plant Growth Promoting Rhizobacteria (PGPR): Emergence in agriculture. World J Microbiol Biotechnol 28(4): 1327-1350.
- Gurikar, Chennappa, Naik MK, Sreenivasa MY (2016) Azotobacter: PGPR Activities with special reference to effect of pesticides and biodegradation. In Microbial Inoculants in Sustainable Agricultural Productivity 1: 229-244.
- Sharma AK (2002) Biofertilizers for sustainable agriculture. Agrobios India, India.
- Behl RK, Sharma H, Kumar V, Narula N (2003) Interactions amongst mycorrhiza, Azotobacter chroococcum and root characteristics of wheat varieties. Journal of Agronomy and Crop Science 189(3): 151-155.
- Maryenko VG (1964) Zavisimost lurozaja kukuruzyl balansa azota aztobacter chroococcum v usbvijah monobakterialznoj kultury (Dependence of maize klyeid and nitrogen balance kon Azotobacter chroococcum in the conditions of monobacterial cultivatiions). Dokt ISHA 99: 399-406.
- Kizilkaya R (2009) Nitrogen fixation capacity of Azotobacter spp. strains isolated from soils in different ecosystems and relationship between them and the microbiological properties of soils. J Environ Biol 30(1): 73-82.
- Mazid M, Taqi AK (2015) Future of bio-fertilizers in Indian agriculture: An overview. International Journal of Agricultural and Food Research 3(3): 10-24.
- Holt JG, Krieg NR, Sneath P, Staley JT, Williams ST (1964) Bergey’s manual of determinative bacteriology. Am J Public Health Nations Health 54(3): 544-546.
- Holt, John G (1994) Bergey’s manual of determinative microbiology. Am J Public Health Nations Health 54(3): 547-550.
- Ehmann A (1977) The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr 132(2): 267-276.
- Vogel AI (2013) A text-book of quantitative inorganic analysis-theory and practice. In: Longmans (Ed.), Green and Co, London, New York, USA.
- Kenichi I, San SY, Nik NA, Toshio O (2012) Ammonia accumulation of novel nitrogen-fixing bacteria. In Biotechnology-Molecular Studies and Novel Applications for Improved Quality of Human Life.
- Salhia B (2010) The effect of Azotobacter chroococcum as nitrogen biofertilizer on the growth and yield of cucumis sativus. Degree of Master. The Islamic University Gaza, Egypt.
- Jiménez, Diego J, José SM, María MM (2011) Characterization of free nitrogen fixing bacteria of the genus Azotobacter in organic vegetablegrown Colombian soils. Braz J l Microbiol 42(3): 846-858.
- Shivaprasad S, Page WJ (1989) Catechol formation and melanisation by na-dependent Azotobacter chroococcum: A protective mechanism for aero adaptation? Appl Environ Microbiol 55(7): 1811-1817.
- Jensen HL (1954) The Azotobacteriaceae. Bacteriol Rev 18(4): 195-214.
- Fankenberger WT, Brunner W (1983) Methods of detection of auxinindole- 3-acetic acid in soil by high performance liquid chromatography. Soil Science Society of America Journal 47(2): 237-241.
- Davies PJ (2013) Plant hormones: Physiology, biochemistry and molecular biology. Springer Science & Business Media, Germany.
- Mirza MS, Ahmad W, Latif F, Haurat J, Bally R, et al. (2001) Isolation, partial characterization, and the effect of plant growth-promoting bacteria (PGPB) on micro-propagated sugarcane in vitro. Plant and Soil 237(1): 47-54.
- Mishra M, Kumar U, Mishra PK, Prakash V (2010) Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicer Arietinum L. growth and germination under salinity. Advances in Biological Research 4(2): 92-96.
- Singh S, Kapoor KK (1994) Solubilization of insoluble phosphates by bacteria isolated from different sources. Environment and Ecology 1(1): 51-55.
- Rodríguez H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growthpromoting bacteria. Plant and Soil 287(1): 15-21.
- Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17(4-5): 319-339.
© 2019 Phyu KE. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






