- Submissions

Full Text
Journal of Biotechnology & Bioresearch
DFT, Quantum Chemical Study and Biological Effects of a Heterocyclic Molecular
Tribak Z*
Department of Science and Technology, Morocco
*Corresponding author:Tribak Z, Department of Science and Technology, Morocco
Submission: January 10, 2019;Published: February 20, 2019

Volume1 Issue3February, 2019
Abstract
In this paper, the title compound 5-chloro-1-(prop-2-yn-1-yl)indoline-2,3-dione (TZP) has been prepared by N-alkylation method with a good yield. The structure of the compound was further confirmed from the 1H NMR and 13C NMR Spectral data and It has been screened for its antibacterial activity. The results revealed that the compound exhibited good to moderate antibacterial activity. Through computational study based on density functional theory (DFT/B3LYP) using basis set 6-31G (d,p) a number of chemical Quantum descriptors were computed to predict the reactivity and the reactive sites on the molecules. The molecular geometry and the electronic properties such as frontier molecular orbital were investigated to get a better insight of the molecular properties. The Molecular electrostatic potential (MEP) for the compound was determined to check their electrophilic or nucleophilic reactivity.
Keywords: 5-Chlorosatin; N-alkylation; Antibacterial effects; DFT; HOMO; LUMO; MEPs
Introduction
5-Chloroisatin derivatives [1] are well known for their versatile therapeutic agents in medicine, exhibiting antimicrobial [2], antiinflammatory [3], antioxidant [4,5], anticancer [6], antibiotic [7], anti-HIV [8], anticonvulsant [9], antitubercular [10] activities and relaxant effects [11]. Density functional theory (DFT) studies have evolved to a powerful and very reliable tool, being routinely used for the determination of various molecular properties [11]. In view of these observations, the aim of the present investigation was to design 5-chloro-1-(prop-2-yn-1-yl)indoline-2,3-dione (TZP) in the search for high expected antibacterial interest against gram positive bacteria Bacillus subtilis, Staphylococcus aureus and Gram-negative bacteria Escherichia coli. Then, a description of the molecular geometry, frontier molecular orbital (HOMO and LUMO), global and local reactivity descriptors and MEP features of the title compound using density functional theory using (DFT/B3LYP) method with the 6-31G (D, P) basis set [12].
Experimental
General
All melting points are uncorrected. 1H-NMR (300MHz) and 13C-NMR (75MHz) spectra were obtained on Bruker equipment using CDCl3 as solvent. Chemical shifts are given in ppm with TMS as an internal reference. J values are given in Hertz. Signals are abbreviated as singlet, s; doublet, d; double-doubles, dd; triplet, t; multiplet, m. Chromatography was performed with silica (mesh) and reactions were monitored by thin layer chromatography (TLC) with silica plates coated with silica gel.
General procedure
5-chloro-1H-indole-2,3-dione (0,4g, 2,20mmol) was dissolved in 15mL of N, N-di methyl formamide (DMF) and 0,5g (3,3mmol) of K2CO3, BTBA (0,1g, 0,3mmol) and 1.2 equivalent of propargyl bromide were added, and the mixture was stirred for 48 hours at room temperature. The reaction progress was monitored by TLC. The solvent was removed in vacuo and co-evaporated with methylene Chloride (CH2Cl2) several times, to remove the remaining traces of DMF. This yielded the product as a red to orange solid. No purification was necessary.
Compound
TZP: 5-chloro-1-(prop-2-yn-1-yl)indoline-2, 3-dione: yield: 88% ; M.P: 166-170 °C; Rf= 0.78 ; 1H NMR (CDCl3) δppm 7.57-7.62 (m, 2H, HAr); 7.12 (d, H, HAr , 3JH-H =6Hz); 4.54 (s, 2H, CH2); 2.34 (t, H, 4JH-H =3Hz); 13C NMR (CDCl3) δppm: 181.55 (C=O); 156.60 (N-C=O); 147.87, 130.07, 118.50 (CQ); 137.80, 125.24, 112.75 (CHAr) ;73.72 (C≡C) ;71.21 (CH); 29.59 (CH2)
Antibacterial activity
Antibacterial screening of compound 5-chloro-1-(prop-2-yn- 1-yl) indoline-2,3-dione (TZp) was determined by a disc diffusion method, against two bacteria Gram-: Pseudomonas aeruginosa, Escherichia coli, and two others Gram+: Bacillus cereus and Staphylococcus aureus using LB medium. Antibacterial activity was carried out according to the method reported by [13] by the determinations of Minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) [14]. The diameter of the inhibition zone around each disc was measured (Table 1).
Computational details
The full geometry optimization of the investigated molecule was carried out by using the Gaussian 09 software [15]. The geometrical parameters were calculated based on the DFT theory with B3LYP hybrid functional and 6-31G (D,P) as basis set. Electronic parameters such as the dipole moment (DM), the energy of the highest occupied molecular orbital and the energy of the lowest unoccupied molecular orbital have been obtained from the log file. In order to obtain a complete image on the chemical potential of the studied compound, we have also calculated the following parameters: energy gap, the chemical hardness
where IP = -EHOMO is the ionization potential and EA = -ELUMO is the electron affinity, the chemical softness [16]
Indeed, the molecular electrostatic potential is very helpful in understanding the net electrostatic effect produced at a point in the space around a molecule by the total charge distribution of the molecule, electrons and nuclei [17]. The electrostatic potential is considered predictive of chemical reactivity because regions of negative potential are expected to be sites of protonation and nucleophilic attack, while regions of positive potential may indicate electrophilic sites [18].
Results and Discussions
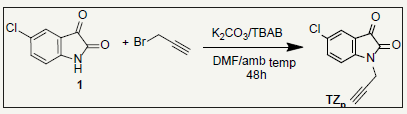
The starting material 5-chloro-1-(prop-2-yn-1-yl) indoline-2,3- dione (TZP) was prepared adopting the reported procedure [19-23]. The 5-Chloroisatin was alkylated with propargyl bromide in DMF as a solvent and anhydrous potassium carbonate and BTBA was added to scheme 1. The structures of the product were confirmed by its spectral data (Figure 1).
Figure 1:Scheme 1.
In vitro Antibacterial Assay
The 5-chloro-1-(prop-2-yn-1-yl) indoline-2,3-dione (TZP) derivative have being screened in vitro for its potency against bacterial strains such as, Bacillus cereus, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa [24]. The antibacterial inhibitions of test compound are expressed as the area of zone of inhibition and summarized in Table 1. The compound 5-chloro-1-(prop-2-yn-1-yl) indoline-2,3-dione (TZP) displays good antibacterial activity (0.625/0.625) against Staphylococcus aureus and moderate antibacterial activity (2.5/2.5) against Escherichia coli. This marked antibacterial activity may be due to the presence of high hydrophobic content of this family of compounds and the indole ring system (Table 1).
Table 1:Antibacterial activity expressed as inhibition zones.

Theoretical and computational DFT Calculations details
The main purpose of quantum chemical descriptors is established by their wide applicability in numerous areas of physical, organic analytical and biomedical chemistry [12]. The optimized structures of the title compound 5-chloro-1-(prop-2- yn-1-yl) indoline-2,3-dione (TZP) were calculated by DFT using B3LYP functional 6-31G (D, P) as basis sets, which used as models to describe the geometric structure [25] (Figure 2).
Frontier Molecular Orbital (FMO)
Figure 2:Optimized molecular structure of TZ1 in different format.

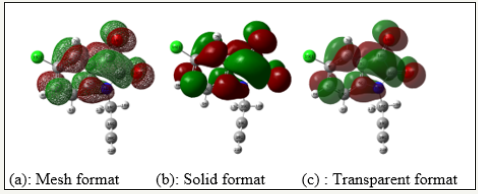
Molecular orbitals and their properties are the most widely used theory by chemists, their electron densities were used for predicting the most reactive position in π-electron systems and also play an important role in the electronic and optical properties, as well as in UV-VIS spectra and chemical reactions [15]. The highest occupied molecular orbital (EHOMO= -6.7608eV) represents the ability to donate an electron, lowest unoccupied molecular orbital (ELUMO= -3.2462eV) as an electron acceptor, represents the ability to obtain an electron, The FMOs of substituted molecule, with B3LYP/6 31G(D,P) method is plotted in (Figure 3 & 4). Therefore, while the energy of the HOMO is directly related to the ionization potential, LUMO energy is directly related to the electron affinity.
Figure 3:Charge distribution of the HOMO molecular orbitals in the optimized TZP in different format.
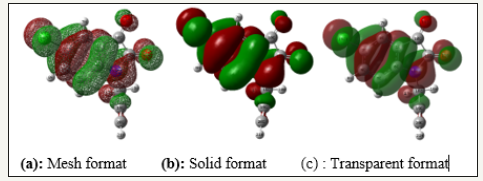
Figure 4:Charge distribution of the LUMO molecular orbitals in the optimized TZP in different format.
The HOMO-LUMO energy gap indicates the chemical reactivity of the molecule whether the molecule is “hard” or “soft”. The compound 5-chloro-1-(prop-2-yn-1-yl) indoline-2,3-dione (TZP) characterized by a small energy gap (ΔEgap=3.5146eV) is known as “soft” molecule which is more polarizable than the hard one because it needs small excitation energies, which influences the biological activity of the molecule [26]. The dipole moment is a parameter that describes the electronic distribution in a molecule and thus its electrostatic interactions with biological macromolecules. According to the energies of the frontier molecular orbitals, several chemical reactivity indices such as electronegativity (χ), total hardness (η) [27] were proposed for understanding the different pharmacological aspects of drug molecule. Hence, the electronegativity contains information about electron transfer, while the total hardness is a measure of the resistance to charge transference [28]. It is concluded that the title compound is stable and reactive. All These descriptors are calculated by B3LYP/6-31G (DP) method and given below (Table 2).
Table 2:Calculated energy values of compound (TZP) by B3LYP/6- 31G(D, P).

Molecular Electrostatic Potential Map
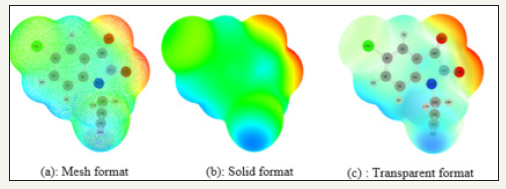
The molecular electrostatic potential (MEP) is used to grasp the molecular interactions, It is a plot of electrostatic potential mapped onto the constant electron density surface. The MEP diagram has been also used to predict chemical reactivity and sites, hence, the negative electrostatic potential corresponds to an attraction of the proton by the aggregate electron density in the molecule (shades of red), while the positive electrostatic potential corresponds to the repulsion of the proton by the atomic nuclei (shades of blue). Potential increases in the order red < orange < yellow < green < blue [29]. In order to identify the reactive sites sensitive to electrophilic and nucleophilic attack [30], the MEP map Figure 4 was calculated by DFT/B3LYP at 631G (D, P) basis set for the optimized structure of 5-chloro-1-(prop-2-yn-1-yl) indoline-2,3-dione (TZP) (Figure 5). The calculated results show that, the MEP surfaces clearly indicate that regions have negative potential are on electronegative oxygen atoms belonging to ketone group and to amide group, these regions being possible active sites for electrophilic attack as well as the regions having positive potential are around the hydrogen atoms, these being the most probable sites for nucleophilic attack.
Figure 5:Molecular electrostatic potential diagram of TZp in different format
Conclusion
In the present study, the 5-chloro-1-(prop-2-yn-1-yl)indoline- 2,3-dione (TZP) has been N-alkylated, synthesized and structurally characterized by elemental analysis, 1H NMR and 13C NMR, it was prepared in good yield. Based on the density functional theory B3LYP/6-31G(D,P) method, the optimized structures, the electronic parameters, i.e. the energy gap, the ionization energy, the chemical hardness, the chemical softness and the Molecular electrostatic potential are theoretically determined. Hence, the calculated HOMO and LUMO energies showed that charge transfer had occurred within the molecule. Molecular electrostatic potential map diagram shows that the negative potential sites are in electronegative atoms (denoted as red color) while the positive potential sites are around the hydrogen atoms (denoted as blue color), the small HOMO-LUMO gap value shows that the molecule is biologically active. Then, the obtained antibacterial activity results indicate that the compound exhibits good to moderate activity against all tested pathogens.
References
- Tribak Z, Skalli MK, Senhaji O, Rodi YK, Haoudi A, et al. (2017) A review on recent advances and applications of 5-chloroisatin and its derivatives in design and synthesis of new organic compounds. American International Journal of Research in Formal, Applied & Natural Sciences 1: 41–50.
- El Sawy E, Mandour A, Mahmoud K, Islam I, Abo Salem H (2012) Synthesis, antimicrobial and anti-cancer activities of some new n-ethyl, n-benzyl and n-benzoyl-3-indolyl heterocycles. Acta Pharm 62(2): 157– 179.
- Fernandes E, Costa D, Toste SA, LimaJLFC, Reis S (2004) In vitro scavenging activity for reactive oxygen and nitrogen species by nonsteroidal anti-inflammatory indole, pyrrole, and oxazole derivative drugs. Free Radic Biol Med 37(11): 1895–1905.
- Estevão MS, Carvalho LC, Ribeiro D, Couto D, Freitas M, et al. (2010) Antioxidant activity of unexplored indole derivatives: Synthesis and screening. Eur J Med Chem 45(11): 4869–4878.
- Talaz O, Gülçin I, Göksu S, Saracoglu N (2009) Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorganic Med Chem 17(18): 6583–6589.
- El-Sawy ER, Mandour AH, El-Hallouty SM, Shaker KH, Abo Salem HM (2013) Synthesis, antimicrobial and anticancer activities of some new n-methylsulphonyl and n-benzenesulphonyl-3-indolyl heterocycles. 1st Cancer update. Arab J Chem 6(1): 67–78.
- Williams RB, Hu JF, Olson KM, Norman VL, Goering MG, et al. (2010) Antibiotic indole sesquiterpene alkaloid from greenwayodendron suaveolens with a new natural product framework. J Nat Prod 73(5): 1008–1011.
- Selvam P, Chandramohan M, De Clercq E, Witvrouw M, Pannecouque C (2001) Synthesis and Anti-HIV activity of 4-[(1,2-dihydro-2-oxo- 3h-indol-3-ylidene) amino]-n(4,6-dimethyl-2-pyrimidinyl)-benzene sulfonamide and its derivatives. Eur J Pharm Sci 14(4): 313–316.
- Singh GS, Singh T, Lakhan R (1998) Synthesis, 13C NMR and anticonvulsant activity of new isatin‐based spiroazetidinones. Chem Inform 29(17).
- Liu, M. Isatin (2018) Derivatives with potential antitubercular activities. Journal of Hetrocyclic Chemistry 55(6): 1263-1279.
- Tribak Z, Chda A, Skalli MK, Haoudi A, Rodi YK, et al. (2018) Theoretical approach using DFT and muscle relaxant effects of 5-Chloroisatin derivatives. Int J Chem Technol 2(2): 105–115.
- Gosav S, Paduraru N, Maftei D, Birsa ML, Praisler M (2017) Quantum chemical study of a derivative of 3-Substituted dithiocarbamic flavanone. Spectrochim Acta Part A Mol Biomol Spectrosc 172: 115–125.
- Tribak Z, Chda A, Skalli MK, Haoudi A, Rodi YK, et al. (2018) Cyclocondensation, characterization and antibacterial activities of novel 5-Chloro-1HIndole-2,3-Dione derivatives. J Appl Microbiol Biochem 2(2): 1–7.
- Tribak Z, El Amin O, Skalli MK, Senhaji O, Kandri, et al. (2016) N-Alkylation methods, characterization and evaluation of antibacterial activity of some novel 5-Chloroisatin derivatives. Int J Eng Res Appl 7(6): 21–24.
- Tribak Z, Sehnaji O, Skalli MK, Haoudi A, Rodi YK, et al. (2017) Experimental and theoretical DFT study on synthesis of novel 5-Chloroisatin derivatives via 1 , 3-Dipolar cycloaddition reactions between Allyl-5-Chloroindoline-2 , 3-Dione and 4-Chlorobenzaldoxime 4(5): 2–7.
- Tribak Z, Skalli M, K.Senhaji O, Kandri, Rodi Y (2017) Synthesis, structural characterization and comparison of experimental and theoretical results by DFT level of molecular structures of 1,2,3-triazoles derived from 5-Chloroisatin. Int J Adv Chem 5(2): 91–95.
- Alkorta I, Perez JJ (1996) Molecular polarization potential maps of the nucleic acid bases. Int J Quantum Chem 57(1): 123–135.
- Politzer P, Murray JS (2002) The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor Chem Acc 108(3): 134–142.
- Tribak Z, Haoudi A, Kandri, Rodi Y, Elmsellem H, et al. (2016) Synthesis and reactivity of new heterocyclic systems derived from 5-Chloro- 1H-Indole-2,3-Dione. Moroccan J Chem 4(4): 1157–1163.
- Tribak Z, Kandri, Rodi Y, A Haoudi, Essassi, et al. (2016) 5-Chloro-1- Methylindoline-2,3-Dione. IUCr Data 1: x160913.
- Tribak Z, Kandri, Rodi Y, Haoudi A, Essassi EM, et al. (2016) 1-Benzyl-5- Chloroindoline-2, 3-Dione IUCr Data 1(6): x160854.
- Tribak Z, Kandri Rodi Y, Haoudi A, Essassi EM, Capet F, et al. (2016) 1-(12-Bromododecyl)-5-Chloroindoline-2,3-Dione. IUCr Data 1(6): x160971.
- Tribak Z, Kandri Rodi Y, Haoudi A, Essassi EM, Capet F, et al. (2016) 1-Allyl-5-Chloroindoline-2, 3-Dione. IUCr Data 1(6): x160862.
- Tribak Z, Amin O, Skalli MK, Senhaji O, Rodi YK, et al. (2017) Synthesis, characterization, and antibacterial activity of some novel 5-Chloroisatin derivatives. Int J Eng Res Appl 7(6): 66–70.
- Tribak Z, Skalli MK, Senhaji O, Rodi, YK (2017) DFT Study and Synthesis of New 1 , 2 , 3-Triazoles Obtained by 1 , 3-Dipolar Cycloaddition Derived 6(7): 1069–1074.
- Saranya M, Ayyappan S, Nithya R, Sangeetha RK, Gokila A (2018) Molecular structure , NBO and homo-lumo analysis of quercetin on single layer graphene by density functional theory. 13(1): 97–105.
- Zhang G, Musgrave CB (2007) Comparison of DFT methods for molecular orbital eigenvalue calculations. The Journal of Physical Chemistry 111(8): 1554–1561.
- Barhoumi M, Lazaar K, Said M (2018) DFT study of optoelectronic and magnetic properties of a novel type perovskites. Chem Phys 513: 120- 128.
- Ali SN (2016) A new approach for studying bond rupture / closure of a spiro benzopyran photochromic material : Reactivity descriptors derived from frontier orbitals and DFT computed electrostatic potential energy surface maps. International Journal of Photoenergy pp. 1–10.
- Piquemal J, Darden TA (2005) Intermolecular electrostatic energies using density fitting. The Journal of Chemical Physics 123(4): 1-10.
© 2018 Tribak Z. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






