- Submissions

Full Text
Innovation in Tissue Engineering & Regenerative Medicine
Plant-Derived Extracellular Vesicles as a Platform for Drug Delivery
Pomatto MAC1,2 and Camussi G1,2*
1EvoBiotech S.R.L. Turin, Italy
2Department of Medical Sciences, University of Turin, Turin, Italy
*Corresponding author:Giovanni Camussi, EvoBiotech S.R.L. Turin, Department of Medical Sciences, University of Turin, Turin, Italy
Submission: January 19, 2023;Published: January 26, 2023

Volume1 Issue5January , 2023
Opinion
Originally described in mammalians, extracellular membrane vesicles were found to be present not only in eukaryotes but also in all life kingdoms including prokaryotes and plants [1]. In eukaryotes, these vesicles were found to be secreted by cells as exosomes when derived from the multivesicular bodies or as ectosomes (also called microvesicles) when derived by budding of plasma membrane [2]. The same cell can originate both types of vesicles and since several of their features (including biogenesis, size, and mechanism of action) are largely superimposable and difficult to distinguish, it has been suggested to collectively call them as Extracellular Vesicles (EVs) [3]. EVs are constituted by a bilayer lipid membrane derived from the cell of origin incorporating molecular messages that can be shared with cells in a paracrine way [4]. Of interest, the membrane of EVs provides protection from enzymemediated degradation of their molecular content, including nucleic acids. It has been shown that EVs may allow a horizontal transfer of functional mRNA naturally carried by EVs as well as exogeneous mRNA incorporated in EVs [5]. Subsequent studies extensively investigated native or engineered EVs as carriers for mRNA, noncoding RNA, and DNA for regenerative purposes [4]. In particular, stem cell-derived EVs were shown to mimic the pro-regenerative activity of the cell of origin based on the transfer of transcripts that target pathways in the injured recipient cells [6]. In order to generate EVs specifically targeting defined pathways, their cargo modification was obtained by genetically engineering of the cell of origin or by various methods of chemically/mechanically induced modification of EV membrane [3,7-10]. Multiloading methods have been also developed enhancing the exploitation of EVs for drug delivery [11] but the manufacturing yield of EVs still represents a limitation to overcome.
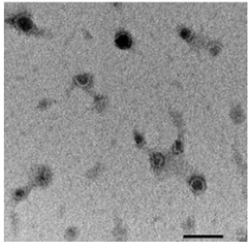
EVs have been described also in plant kingdom [12]. Little is known about the mechanism of biogenesis and function, but their structures and sizes are very similar to the ones of eukaryotes [13] (Figure 1). Plant-derived EVs can be extracted from different parts of plants and they are particularly enriched in plant-derived juices. Plant EVs, being an extractive product, can be a good option for scalable EV production for drug delivery purposes. In particular, EVs derived from edible plants are non-toxic and, due to the mechanism of oral tolerance, they are also not immunogenic [13-15]. Edible plant-derived EVs have been shown to play a role in a crosstalk between the plant and eukaryotes kingdoms [16]. In humans, plant EVs absorbed through the intestinal tract may transfer molecules that modulate pathways in the recipient organisms with influence on health [16]. The properties of plant EVs to protect nucleic acids (microRNAs, small interfering RNAs, RNAs, DNAs), to reduce toxicity of drugs as well as to improve the absorption of poorly soluble compounds (for instance curcumin and liposoluble vitamins) represent key factors to make them an optimal drug delivery platform [17-21].
Figure 1:Transmission electron microscopy of EVs derived from edible plant (Citrus sinensis). Scale bar 100nm.
Using a proprietary technique of EvoBiotech (WO2022152771A1), we engineered EVs obtained from Citrus sinensis juice with SARS-CoV-2 mRNA coding for S1 subunit, Full Spike and N proteins to develop nucleic acid based vaccines. Our data demonstrated the functionality of edible plant-derived EVs as drug carrier for mRNA vaccines and their ability to induce immune stimulation direct against the specific antigen. Previous studies supported the use of EVs as possible vaccine carrier demonstrating that EVs derived from engineered cell lines may express a recombinant S protein on their surface suitable for inducing an immune response [22]. Despites difficulties in manufacturing scalability, EV-based vaccines have advantages in conferring long lasting immunization and lower toxicity than synthetic nanoparticles. In addition, EVs may present the viral antigens in their natural configuration to the immune system [23]. In this context, plant-derived EVs are superior to those derived from cultured cells being a natural, abundant and easily extractable product, more suitable for mRNA delivery in clinic. We found that, after incorporation into plant EVs, mRNA was stable in chemical stress condition and storable at room temperature after lyophilization. Plant EVs conferred resistance to mRNA molecules against RNA degradation and simulated gastric juice. The immunization of multiple rodent models (mice and rats) via different routes including intramuscular, oral and intranasal elicited a humoral and T cell mediated immune response. Of interest, the oral and intranasal administration not only triggered IgM and IgG but also an IgA mucosal immune response.
Conclusion
In conclusion, the development of an EV based anti-COVID mRNA vaccine may represent a prototype for a platform of therapeutic delivery of nucleic acids. The stability and bioavailability of nucleic acids incorporated in edible plant EVs suggest that this natural extractable product may represent a good option for drug delivery, especially suitable for oral administration.
Conflict of Interest
Authors are associated with EvoBiotech s.r.l. and named as inventors in EV related patents.
References
- Ratajczak J, Wysoczynski M, Hayek F, Janowska WA, Ratajczak MZ (2006) Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20(9): 1487-1495.
- Raposo G, Stoorvogel W (2013) Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol 200(4): 373-383.
- Gould SJ, Raposo G (2013) As we wait: Coping with an imperfect nomenclature for extracellular vesicles. Journal of Extracellular Vesicles 2:
- Bruno S, Kholia S, Deregibus MC, Camussi G (2019) The role of extracellular vesicles as paracrine effectors in stem cell-based therapies. Adv Exp Med Biol 1201: 175-193.
- Deregibus MC, Cantaluppi V, Calogero R, Iacono ML, Tetta C, et al. (2007) Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110(7): 2440-2448.
- Bruno S, Chiabotto G, Favaro E, Deregibus MC, Camussi G (2019) Role of extracellular vesicles in stem cell biology. Am J Physiol Cell Physiol 317(2): C303-C313.
- Pomatto MAC, Bussolati B, D'Antico S, Ghiotto S, Tetta C, et al. (2019) Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol Ther Methods Clin Dev 13: 133-144.
- Sadeghi S, Tehrani FR, Tahmasebi S, Shafiee A, Hashemi SM (2023) Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 7: 1-25.
- Liao W, Du Y, Zhang C, Pan F, Yao Y, et al. (2019) Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater 86: 1-14.
- Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci 9.
- Xu M, Yang Q, Sun X, Wang Y (2020) Recent advancements in the loading and modification of therapeutic exosomes. Front Bioeng Biotechnol 8: 586130.
- Feng J, Xiu Q, Huang Y, Troyer Z, Li B, et al. (2023) Plant derived vesicle-like nanoparticles as promising biotherapeutic tools: Present and future. Adv Mater e2207826.
- Fan SJ, Chen JY, Tang CH, Zhao QY, Zhang JM, et al. (2022) Edible plant extracellular vesicles: An emerging tool for bioactives delivery. Front Immunol 13: 1028418.
- Cui Y, Gao J, He Y, Jiang L (2020) Plant extracellular vesicles. Protoplasma 257(1): 3-12.
- Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H, et al. (2013) Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun 4: 1867.
- Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, et al. (2014) Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res 58(7): 1561-1573.
- Gioia S, Hossain MN, Conese M (2020) Biological properties and therapeutic effects of plant-derived nanovesicles. Open Medicine 15(1): 1096-1122.
- Bonifacio BV, Silva PB, Ramos MA, Negri KM, Bauab TM, et al. (2014) Nanotechnology-based drug delivery systems and herbal medicines: A review. Int J Nanomed 9: 1-15.
- Sharma M (2014) Applications of nanotechnology based dosage forms for delivery of herbal drugs. Res & Rev: J Pharm Nanotechnol 2(1): 23-30.
- Rome S (2019) Biological properties of plant-derived extracellular vesicles. Food Funct 10(2): 529-538.
- Yang C, Zhang M, Merlin D (2018) Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B 6(9): 1312-1321.
- Kuate S, Cinatl J, Doerr HW, Uberla K (2007) Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology 362(1): 26-37.
- Yoo KH, Thapa N, Kim BJ, Lee JO, Jang YN, et al. (2022) Possibility of exosome‐based coronavirus disease 2019 vaccine. Mol Med Rep 25(1): 26.
© 2023 Camussi G. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






