- Submissions

Full Text
Innovation in Tissue Engineering & Regenerative Medicine
Transplantation of Autologous Bone Marrow- Derived Stromal Cells in Type 2 Diabetes: A Pilot Study
Shraddha Singh Gautam1, Lipi Singh1, Pawan Sharma2, Punit Prabha1, Sumit Saha1, Shiv Raghav1, Rajkumar Maurya1, Sanjeena Khatun1 and Sachin Kadam1*
1Advancells Group, India
2Institute of Brain and Spine, India
*Corresponding author:Sachin Kadam, Advancells Group, NOIDA, UP 201301, India
Submission: December 04, 2019;Published: December 11, 2019

Volume1 Issue4December, 2019
Abstract
Type 2 Diabetes is a debilitating metabolic disorder which is also the seventh leading cause of death worldwide. Current therapeutic regimes to date have failed to achieve significant long-term glycemic control even with intensive insulin therapy as revealed by deregulated Hb1Ac and C-peptides levels. In the current study, we have evaluated the effect of regenerative cellular therapy for functional recovery from Diabetic pathophysiology. 10 patients with a median age of 51 years were selected for the study and subjected to bone marrow isolation. These samples were processed under sterile conditions for the enrichment of mononuclear cells (BM MNCs) from bone marrow. After strict quality control and characterization of cells, 2 x 106 cells/kg of BM MNCs were infused back into the patient through the anterior pancreaticoduodenal artery. We performed an evaluation of clinical parameters like Body Mass Index, Fasting Plasma Glucose, Fasting Plasma Insulin, HbA1c and C-peptide levels, and followed up the patients for 12 months. Our study showed a reduction in insulin dependency by ≥ 50%.
Keywords: Type 2 diabetes; Pilot study; Bone marrow; BM MNCs; Autologous; Regenerative medicine
Introduction
Diabetes Mellitus (DM), a chronic metabolic disorder, has become a global epidemic affecting 463 million people worldwide and causing substantially devastating co-morbidities and complications, like diabetic neuropathy, cardiovascular disorders, diabetic nephropathy, eye disorders, etc. With a current count of 77 million diabetic people, which is expected to rise to 134.2million by 2045, India is all set to be the capital of diabetes [1]. Indians appear to have a higher disposition towards Type 2 DM (T2DM) than Type 1 DM (T1DM), with its prevalence rate surpassing 12.1% among the Indian population [2]. T2DM is principally outlined by insulin resistance and beta (β) cell dysfunction, leading to hyperglycemia. Apparently, β-cell destruction could begin 12 years before its prognosis and continues to be deteriorated throughout the life of an individual. This progressive nature of β-cell destruction and their functional impairment can lead to poor glycemic control and development of systemic complications [3]. It has been evidently suggested that by gaining optimum glycemic control, it is possible to delay the disease progression [4] as well as the onset of other severe microvascular and macrovascular diabetes induced complications.
Conventional therapeutic regimen available till date [5,6] are intended either to optimize insulin resistance or to rule-out insulin deficiency and hence long-term glycemic control has not been significantly achieved with insulin therapy in a number of initial studies, stating its importance. However, in the present clinical study, these have been recounted to confirm that even with intensive insulin therapy, some patients presented remarkable glycemic fluctuations; indicating poor control of HemoglobinA1C (HbA1C) and reduced C-peptide levels as the fundamental sources of deteriorating glycemic control, further affecting endogenous β-cell function. Regenerative medicine is the latest branch of medical science that has been cropping up in order to deal functional replacement of diseased tissue/cells to with healthy one [7]. Stem cells are the unique fundamental cells with the ability to differentiate into cells of different lineages opening up new avenues in offering effective long-lasting treatment profile, which can trigger cellular regeneration and facilitate functional restoration by reversing overall pathophysiology of T2DM patients. A myriad of data is been accumulated from different in vivo as well as in vitro studies, directly or indirectly acknowledging the robust nature of these stem cells, which can be well enslaved to reverse the damage, associated with the condition. In spite of these studies, very few studies have confirmed their presence from bench to bedside, due to ethical as well as translational considerations [8,9]. Thus, in order to employ these strategies as effective salutary substitutes, several additional rationales should be emphasized, in order to develop a safe and feasible approach to treat T2DM patients.
Various clinical studies have confirmed the potential of many different types of stem cells to differentiate into islet producing β-cells along with a significant reduction in the blood glucose level [10,11] but the advantages of multipotent nature, ease of isolation, large availability and ethical exemption of bone marrow derived mononuclear cells (BM MNCs) justify them as the most acknowledged prospective nominees for treating complex disorders, like diabetes. Apparently, many clinical trials have been conducted to evaluate the probable tendency of these cells in maintaining glycemic control and improving pancreatic function; along with their role in reducing HbA1C and C-peptide levels to an appreciable level. However, long term management with these clinical outcomes is still a debatable issue. Thus, the current study is an open labeled randomized trial conducted in order to evaluate safety, feasibility and efficacy in maintaining long term management of glycemic control post autologous BM MNCs treatment.
Materials & Methods
Study design
The Pilot clinical study was approved by the institutional ethical committee of Institute of Brain and Spine (IBS), India. Written informed consents were obtained from perspective and participating patients before study.
Patient selection
A pilot study was conducted with 10 patients (8 males and 2 females). Selection of patients diagnosed with T2DM, was done strictly according to an inclusion and exclusion criteria set. In brief, patients with age 45 to 55 years, with a history of ≤ 7 years of diabetes and insulin dependent treatment (Insulin dose ≥ 0.4 IU/kg/Day) for maintaining blood glucose function, HbA1C levels between 8-8.6%, and with a willingness to participate in the study were included. Patients with severe pancreatic malignancy, congenital pancreatic abnormalities, endocrinal metabolic diseases, pregnancy, and acute and/or chronic infections, were excluded from the study.
Patient examination and baseline evaluation
All test subjects/patients underwent pre-study assessment to obtained baseline reference values. All the patients underwent clinical assessments regarding glycemic control before commencing the pilot study. The parameters that were checked included blood pressure, body mass index (BMI), fasting and postprandial blood sugar levels by electrochemiluminescence immunoassay analyzer (ECLIA) (Assay sensitivity 0.2μU/mL, Coefficient of variation 1.5% for intra-assay) (Elecsys 2010, Roche Diagnostics, GmbH, Germany). All the participants were asked to stop oral hypoglycemic drugs as well as insulin supplements for 48h. For the model assessment of insulin resistance and beta cells function, fasting plasma insulin and fasting blood glucose levels were rectified using Homeostatic Model Assessment (HOMA) participants were asked to monitor their five-point glucose profiling using glucometer (Abbott, India). These assessments were targeted to be between 70-130mg/dL for fasting plasma glucose levels and ≤180 mg/dL for postprandial. The HbA1C level was measured through automated NycoCard HbA1C card reader (Alere, USA; Normal Reference Range: 3.8-5.9%). A glucagon stimulated C-Peptide secretion was evaluated in fasting state after intravenous infusion of glucagon (1mg/vial, GlucaGen, Novo Nordisk, Denmark). Blood samples were collected before 15 min of infusion, immediately after infusion and 15min post infusion. C-peptide estimation was done with the help of ECLIA (Normal Reference Range: 1.1-4.4ng/mL, assay sensitivity 0.01ng/mL).
Physical exercise & nutritional diet suggested
All the participants were advised 30 min of brisk walking every day; the schedule for the same was recorded in each visit. The participants were offered an individualistic nutritional plan with proportionate mix of all the vital ingredients, depending upon eating preferences. An importance of balance diet was reinforced during every follow up, and the changes in the same were recorded for further analysis.
Collection of human bone marrow
Bone marrow was extracted under local anesthesia from the posterior superior iliac crest by multiple aspirations in the operation theatre under aseptic conditions. Approximately 70 to 100 ml of bone marrow per patient was collected in a collection bag containing sufficient amount of anticoagulant by a qualified surgeon. The collected bone marrow samples were then immediately transferred to the processing facility under controlled temperature of 2- 80 C in a specimen transport box. After collection, samples were checked for in-house quality control parameters, such as viral or bacterial infections. All samples were processed in the cGMP class 10,000 cleanroom under strict sterile environment.
Isolation and enrichment of mononuclear cells isolation from bone marrow
After marrow aspiration, the collected bone marrow was passed through 70μ blood filtration system (Haemonetics, USA). Pre and post processing, bone marrow aliquots were collected separately for quality control analysis. The marrow was processed under a closed system with sterile processing unit of SEPAX 2.0 (Biosafe, Switzerland) allowing automated separation of cellular components as per the manufacturer’s instructions. The final product volumes were maintained between 7-8mL.
Quality control analysis
All the nucleated cells from bone marrow were counted using Sysmex KX-21N cell counter (Sysmex Corporation, Kobe, Japan) whereas Trypan Blue Exclusion Test of cell Viability was performed to confirm the cell viability. The bacterial Endotoxin test was performed to by the gel clot technique (LAL, Pyrogen Ultra, Lonza, Switzerland) to detect post procedure Endotoxin unit.
Cell surface marker characterization by flow cytometric analysis
The isolated BM MNCs were subjected to the Fluorescent Activated Cell Sorting (FACS) Analysis, using six colored standard procedure on FACSCaliburTM (BD Biosciences). BM MNCs pellet containing approximately 5x 105 cells were fixed in chilled 70% ethanol, and incubated in mouse anti-human FITC/PE conjugated antibodies (1:100 dilution) against CD29, CD34, CD 45, CD90 and CD 133 [12,13] for 1h on ice (all antibodies were purchased from Becton Dickinson, San Diego, CA, USA). Finally, the cells were counted using a flow cytometry laser at 488nm, and the data were analyzed using BD CellQuestTM Pro software.
Mode of infusion
BM MNC at a cell density of 2x106 cells/kg body weight were infused into the patients’ anterior pancreaticoduodenal artery via 5F catheterization. The transfusion lasted for 30min, and the patients were discharged after 7h of observation period. Patients were monitored for approximately 24h for any immediate complications. All the patients were assessed for post targeted stem cells injections after 12, 24, 36 and 48 weeks. All the patients were advised to continue the routine medication for diabetes including insulin. The dose of insulin was subjected in accordance with the blood glucose levels.
Post procedure assessment
All the participants were followed up for 12 months with at least one follow-up in every 3-month interval. During the follow up period, all the patients were assessed for body weight, blood glucose level, urine and blood analysis, and blood biochemistry.
Statistics
All the data were expressed as mean, median and ± standard deviation; unless otherwise specified. The linear regression analysis was used to correlate association between two variables.
Results and Discussion
A total of 10 adult patients (8 men and 2 women) with T2DM were included in this present study. The pilot study was carried out to evaluate the safety and efficacy of autologous bone marrow transplantation in T2DM cases. The complete anthropometric, body composition, and biochemical measurements were religiously recorded in the study files. A rundown data about the clinical evaluation of patients baseline demographical as well as biochemical parameters are presented in Table 1. Accordingly, the median age of the patients that had been selected for the study was 51 (45-55) years with an average history of diabetes as 4.3 (2.82- 5.78) years. Out of 10 patients, four had reported hypertension; and rest had no other severe indications during or after the treatment. All the patients were on twice-daily premixed insulin, and the average dose that had been administered was 0.53 (0.46-0.6) IU/ Kg/Day. The average baseline HbA1C was 8.48% (8.12-8.84%).
Table 1:Detailed assessment of baseline clinical and demographical parameters.
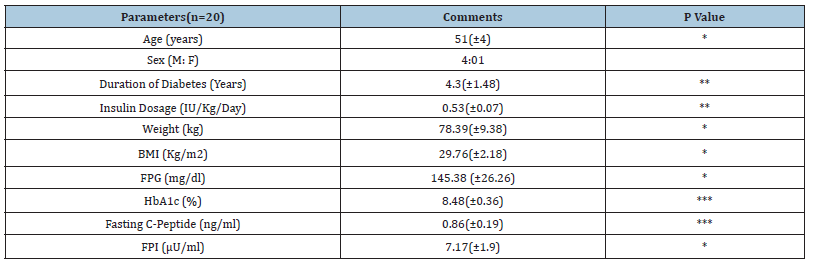
*Non-significant **Significant ***Very significant
BMI-Body Mass Index; FPG-Fasting Plasma Glucose; FPI-Fasting Plasma Insulin; HbA1C-Hemoglobin A1C
Table 2:Aspiration and cell count characteristics of BM MNCs.
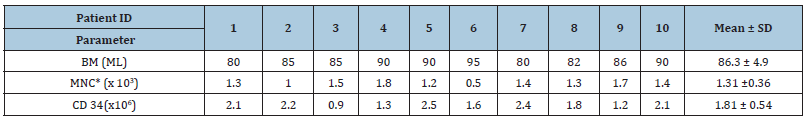
*MNC- Mononuclear Cells
As per the study, all the patients underwent BM MNCs therapy and were assessed on a long-term basis, for 12 months, post treatment. The patients were infused with enriched mononuclear cells from Bone Marrow. The average volume of bone marrow collected from all the 10 patients was 86.4(81.74-91.06) mL. After sterile extraction and enrichment of total MNCs from the bone marrow, the cells were infused back into the patients through anterior pancreaticoduodenal artery via 5F catheterization [14]. Average CD34+ cells infusion per patients was 2x106 cells/kg body weight (Table 2). BM MNC Immunophenotyping results are shown in Figure 1. FACS analysis clearly shows the presence of mixed population of hematopoietic and mesenchymal stromal cells as evidence by presence of CD29, CD34, CD45, CD90, CD133 on isolated BM MNCs surface. HLA-II marker was found negative during the FACS analysis. Glycosylated Hemoglobin (HbA1c) is less susceptible to diet, exercise, and other external factors; this makes HbA1c a better blood glucose monitoring system (Figure 2A). Hence, we have checked the Hb1c level pre therapy and every 3 months post therapy (Figure 2B). Levels of Hb1c were significantly reduced during the 12 months follow up period post therapy, indicating fair control over blood glucose levels.
Figure 1:Representative data of Immunophenotyping of BM MSCs isolated from patients.
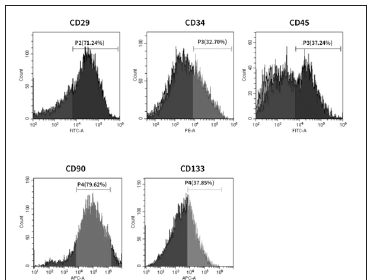
Figure 2:Changes in Body weight index.
A. Glycosylated Hemoglobin
B. Fasting Blood Sugar
C. and Postprandial Blood Sugar
D. before and after treatment
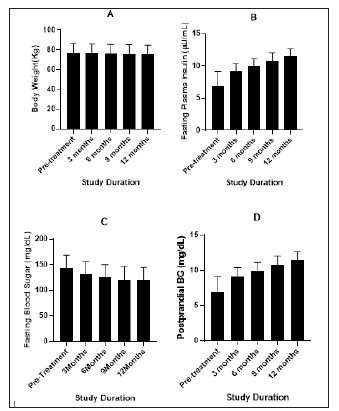
The link between weight and T2DM is very strong. An overweight/obese person is more likely to develop T2DM. In previous studies it has been observed that weight loss or being weight stable with little weight variability early after diabetes diagnosis, are associated with better glycemic control [15]. Post BM MNC therapy it was observed that all the patients were able to maintain their weights with an average reduction of 6.5kg during the follow up period of 12 months, indicating active metabolism (Figure 2A). This is a good indication as weight loss in T2DM patients improves glycemia and lower the requirements for conventional. glucose-lowering medicines, Improvement of glycemic control further affects increments in peripheral insulin sensitivity with improvements in insulin signal transduction at the cellular level, with better insulin secretory responses, and reduction in hepatic glucose production [16]. The correlation coefficients between the change in the plasma glucose level (both fasting and postprandial) and glycemic control is directly proportional to each other. All body composition parameters like body weight, Blood glucose level, insulin dependence has decreased significantly from baseline to month 3 and then to month 6 and further over the period of 12 month. (Figure 2C) clearly shows the progressive reduction in fasting blood glucose level from third month (7.6%) onwards of the therapy till 12-month (15.73%) period. The post prandial blood glucose level also showed significant reduction to 18.99% at 12-month post therapy indicating fair control over blood glucose (Figure 2D).
Figure 3:Figure 3: : Changes in C-peptide level.
A. Fasting Plasma Insulin
B. Insulin Dosage requirement
C. before and after treatment
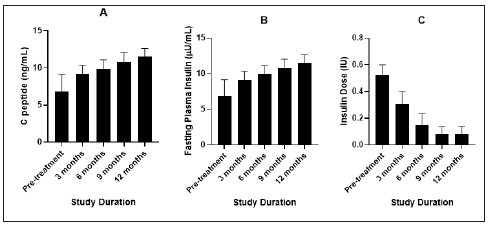
Figure 4:Comparative evaluation of study parameters.
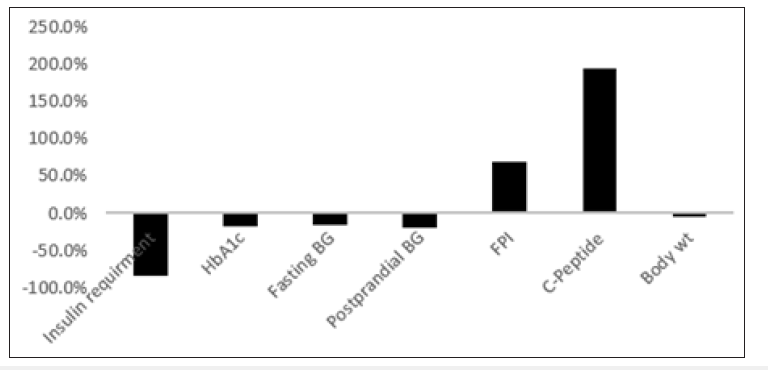
Insulin is a hormone secreted by pancreatic beta islets in response to glucose. C-peptide is a byproduct created when insulin is produced. Measuring the amount of C-peptide in blood indicates how much insulin is being produced (Figure 3A,3B & 3C). Generally, higher values of C-peptide production indicate the higher amount of insulin in circulation. Similarly, lower c-peptide values have been associated with poorer glycemic control and hence increased HbA1c values [17,18]. Post study evaluation of patients clearly indicate similar findings with direct co-relation between c-peptide and glycemic control (Figure 4). C-peptide level can be the direct indicative for use of insulin therapy and also any other futuristic individual therapies. This shows that alongside HbA1c, C-peptide level determination is very helpful in diagnosing and monitoring the diabetes [19].
After stem cells therapy, 80% patients were observed to be fulfilling a primary objective of the study i.e., reduction of insulin dependency by≥ 50%, and hence were referred to as responders for readers reference; whereas the rest 20% patients were referred to as non-responders. In this study, we postulate that adult BM MNCs possess the capability for islet cell regeneration, thus leading to functional restoration of insulin production. Our processed cells demonstrated strong positivity for Mesenchymal as well as Hematopoietic stem cells markers during immunophenotyping assay. After a 12-month follow-up, patients showed significant reduction in HbA1C levels thus showing controlled regulation of blood glucose. There was also weight loss reported in patients signifying active metabolism. Overall, 80% patients showed improvement in clinical parameters (Figure 4). Further, there were no adverse effects or complications observed in our patients. Our study fulfilled the primary objective of achieving reduction of insulin dependency by ≥ 50% and also ameliorated insulin resistance in patients.
Although, the results of the 12-month follow-up study showed significant glycemic control over the period and near normalize parameters (Figure 4), this study has few limitations. The most important one is the geographical distribution of patients which limit the firsthand data collection during given time point. Thus, we had to rely on the data provided by few patients this made study design more retrospective and not a prospective which have allowed us to establish a causal-effect relationship between the parameters evaluated.
Conclusion
T2DM arises from dysfunction of insulin production by pancreatic cells leading to elevated glucose levels in blood. The Patients are dependent on exogenous insulin to circumvent the resultant hyperglycemia. Diabetes has also been associated with several co-morbid conditions such as Diabetic retinopathies, renal and eye disorders, and cardiovascular diseases [20,21]. Till date, conventional therapeutic strategies have been unable to achieve glycemic control and only provides symptomatic cure. Recently, cell-based therapies have been investigated as a promising treatment for T2DM. In this pilot study, we investigated the efficacy of autologous infusion of Bone marrow derived mononuclear cells in 10 T2DM patients. MNC administration has been reported to assist neo-vascularization and angiogenesis in Diabetic patients earlier [22,23]. Our results have shown promising clinical outcomes which can improve the quality of life for T2DM patients. Larger scale clinical studies in future will validate the accuracy and comprehensively assess the efficacy of our treatment regime.
Acknowledgement
This study was funded by intramural grant from Advancells Group. Authors are thankful to Dr. Sachin Kandhari for extending all the required facility to conduct the study at Institute of Brain and Spine, New Delhi, India.
Conflict of Interest
All the authors have no conflict of interest.
References
- International Diabetes Federation (2019) IDF Diabetes Atlas (9th edn), Watermael-Boitsfort, Belgium.
- Mohan V, Pradeepa R (2009) Epidemiology of diabetes in different regions of India. Health Administrator 22(1-2): 1-18.
- Cerf ME (2013) Beta cell dysfunction and insulin resistance. Frontiers in Endocrinology 4: 37.
- Skyler JS (2004) Effects of glycemic control on diabetes complications and on the prevention of diabetes. Clinical Diabetes 22(4): 162-166.
- Pfeiffer AF, Klein HH (2014) The treatment of type 2 diabetes.Dtsch Arztebl Int 111(5): 69-81.
- Donner T, Sarkar S (2019) Insulin - pharmacology, therapeutic regimens, and principles of intensive insulin therapy. Endotext [Internet], South Dartmouth (MA): MDText.com.
- Mao AS, Mooney DJ (2015) Regenerative medicine: Current therapies and future directions. Proceedings of the National Academy of Sciences of the United States of America 112(47): 14452-14459.
- Liu Y, Guo LB, Xu JK (2016) Amniotic stem cell transplantation therapy for type 2 diabetes: 3 years' follow-up report. European Review for Medical and Pharmacological Sciences 20(18): 3877-3885.
- Liu X, Zheng P, Wang X, Dai G, Cheng H, et al. (2014) A preliminary evaluation of efficacy and safety of Wharton’s jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Research & Therapy 5(2): 57.
- Solis MA, Moreno Velásquez I, Correa R, Huang L (2019) Stem cells as a potential therapy for diabetes mellitus: A call-to-action in Latin America. Diabetology & Metabolic Syndrome 11: 20.
- Cheng SK, Park EY, Pehar A, Rooney AC, Gallicano GI, et al. (2016) Current progress of human trials using stem cell therapy as a treatment for diabetes mellitus. American Journal of Stem Cells 5(3): 74-86.
- Pham H, Tonai R, Wu M, Birtolo C, Chen M (2018) CD73, CD90, CD105 and cadherin-11 RT-PCR screening for mesenchymal stem cells from cryopreserved human cord tissue. International Journal of Stem Cells 11(1): 26-38.
- Wu CY, Zhang LS, Zhang YF, Chai Y, Yi LC, et al. (2005) In vitro inducing differentiation of bone marrow mononuclear cells of chronic myeloid leukemia. Ai Zheng 24(4): 425-431.
- Zang L, Hao H, Liu J, Li Y, Han W, et al. (2017) Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetology & Metabolic Syndrome 9: 36.
- Aucott LS, Philip S, Avenell A, Afolabi E, Sattar N, et al. (2016) Patterns of weight change after the diagnosis of type 2 diabetes in Scotland and their relationship with glycaemic control, mortality and cardiovascular outcomes: A retrospective cohort study. BMJ Open 6(7): e010836.
- Grams J, Garvey WT (2015) Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy and bariatric surgery: Mechanisms of Action. Curr Obes Rep 4(2): 287-302.
- Kuhtreiber WM, Washer SLL, Hsu E, Zhao M, Reinhold P, et al. (2015) Low levels of C-peptide have clinical significance for established type 1 diabetes. Diabet Med 32(10): 1346-1353.
- Lachin JM, McGee P, Palmer JP, DCCT/EDIC Research Group (2014) Impact of C-peptide preservation on metabolic and clinical outcomes in the diabetes control and complications trial. Diabetes 63(2): 739-748.
- Leighton E, Sainsbury CA, Jones GC (2017) A practical review of C-peptide testing in diabetes. Diabetes Ther 8(3): 475-487.
- Sayin N, Kara N, Pekel G (2015) Ocular complications of diabetes mellitus. World Journal of Diabetes 6(1): 92-108.
- Leon BM, Maddox TM (2015) Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World Journal of Diabetes 6(13): 1246-1258.
- Bhansali A, Upreti V, Walia R, Gupta V, Bhansali S, et al. (2014) Efficacy and safety of autologous bone marrow derived hematopoietic stem cell transplantation in patients with type 2 DM: A 15 months follow-up study. Indian Journal of Endocrinology and Metabolism 18(6): 838-845.
- Wang L, Zhao S, Mao H, Zhou L, Wang ZJ, et al. (2011) Autologous bone marrow stem cell transplantation for the treatment of type 2 diabetes mellitus. Chinese Medical Journal 124(22): 3622-3628.
© 2019 Carlotta Pucci. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






