- Submissions

Full Text
Innovation in Tissue Engineering & Regenerative Medicine
Innovative Potential of Periodontal Ligament Cell Sheet Engineering in Functional Implant Therapy
Isao Ishikawa1*, Kaoru Washio1, Kosei Yano1, Kengo Iwasaki2 and Yuka Tumanuma3
1 Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, Japan
2 Institute of Dental Research, Osaka Dental University, Japan
3 Department of Periodontology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU), Japan
*Corresponding author:Isao Ishikawa, Institute of Advanced Biomedical Engineering and Science Tokyo Women`s Medical University, 8-1 Kawada-cho, Shinjuku-ku, Tokyo, 162-8666, Japan
Submission: October 03, 2018;Published: October 09, 2018

Volume1 Issue2October 2018
Abstract
Implant therapy is now one of the important dental treatments for reconstruction of oral function. Recently, increasing attention has been paid to several complications related to implant treatment, including inflammatory peri-implantitis. To solve these implant complications and restore missing functions of dental implants as compared to the natural tooth, several efforts have been performed to construct periodontal tissues around the implant, mimicking the anatomical structure of a natural tooth. In this mini review, we will first overview the previously reported studies to form periodontal tissues around the dental implant. Secondly, we will demonstrate our recent trial to construct a periodontium-based implant using cell sheet engineering. We succeeded in the formation of cementum- and periodontal ligament-like tissue around a titanium implant by transplanting a periodontal ligament cell sheet with a surface-modified implant in dogs. Finally, as future directions, some considerations that may be useful to obtain more stable periodontal apparatus structures around implants will be discussed, including artificial formation of the cementum on the titanium surface. This mini review aims to summarize previous trials and recent progress in the formation of implants with periodontium and to provide further insights for innovative implant therapy.
Keywords: Implant; Periodontal ligament; Cell sheet; Cementum
Introduction
Skin
Currently, dental implant therapy is considered as one of the most important procedures in dentistry. Especially osseointegrated implants, which were established by Branemark et al. [1], have been used worldwide as a reliable and consistent procedure. However, recent surveys revealed that peri-implantitis was considered as a major and growing problem in implantology [2-4]. They reported that peri-implantitis occurred in 28-56% of subjects after 5-10 years of use. As one of the causes of periimplantitis, it has been suggested that peri-implant soft tissue may have an impaired defense capacity against exogenous irritation due to lack of periodontal supra-crestal ligaments [5]. Dental researchers are now asked to solve this problem. Giannobile [6] has suggested that the presence of a periodontal ligament (PDL) allows for a more dynamic role beyond the ankylosed dental implants. Implants with periodontal ligament may improve the function of ankylosed implants by providing various biological characteristics of periodontium, such as durability against bacterial challenge and acting as a buffer and sensor of biting force.
Previous efforts to create a new PDL formation around implants
The possibility of new ligament formation around implants has been investigated. In 1990, Buser et al. [7] found accidentally the formation of PDL around titanium implants at 12 months after implantation in monkeys. Closing to the retained root, PDL was formed around a large portion of the adjacent implant. This initial observation suggested the possibility to achieve and anchorage of dental implant with periodontal ligament. Subsequently, Choi [8] reported that at 3 months after implantation with cultured autogenous PDL cells, a layer of cementum-like tissue with inserting collagen fibers has been observed on some implant surfaces. In addition, various efforts have been performed to form periodontal tissues around titanium implants using an orthodontic technique [9] or dentin chamber model [10]. A cell culture method around the implant utilizing a bio-engineering approach was investigated. In 2010, Gault et al. [11] demonstrated that a tissue-engineered PDL around implants promoted the formation of a biological interface between the implant and PDL cells. They used a bioreactor to culture the PDL cells on a titanium implant and then transplanted them to the osseous defect. They claimed that the regenerated tissues were arranged similarly to naturally occurring PDL. This report was the first to successfully demonstrate ligament implant integration. In 2011, Lin et al. [12] reported that autologous PDL progenitor cells formed organized periodontal tissues on titanium implants in rats. They used the Matrigel as a scaffold to create a closed contact of cells to the titanium surface. They reported that Matrigel coated implants exhibited periodontal regeneration with a thin layer of cementum-like tissue. Oshima et al. [13] reported that hydroxyapatite (HA)-coated dental implants enveloped with embryonic dental follicle were transplanted into the mandible in rats. They found a novel fibrous connection to the implant. Recently, Nakajima et al. [14] showed a novel bio-engineering method for a functional bio-hybrid implant in mice. HA-coated titanium implants were at first implanted into bony holes. Approximately one month later, the implants were extracted with the surrounding alveolar bone tissue. The bone-attached implants were wrapped with two or three pieces of PDL tissue and then transplanted again into bony holes of the mice. They concluded that the functional biohybrid implant had potential for clinical use. Reliable procedures for obtaining close and stable interfaces between the implant and PDL cells or tissues have been explored, but practical periodontal attachments on the implant surface, which can be applied in clinical settings, have not been established.
Cell sheet engineering
Okano et al. developed a novel innovative tissue culture dish using surface-grafted temperature-responsive polymers. The polymer-grafted surface can control cell adhesion through changes in culture temperature [15]. Cultured cells are harvested from the dishes as a sheet accompanied with intact extracellular matrix without any disturbance to the cells [16]. They named this unique cell sheet formation technique as “cell sheet engineering”. Since then, cell sheet engineering has been applied to a wide variety of regenerative therapies in humans, such as cartilage, esophagus, lung, heart, eye, and periodontal tissues [17-22]. All of these studies demonstrated that cell sheet engineering is a powerful tool for cell transplantation, and now the temperature-responsive culture dish is one of the most widely used methods for cell transplantation in regenerative studies.
In vivo periodontium formation around titanium implants using cell sheet engineering
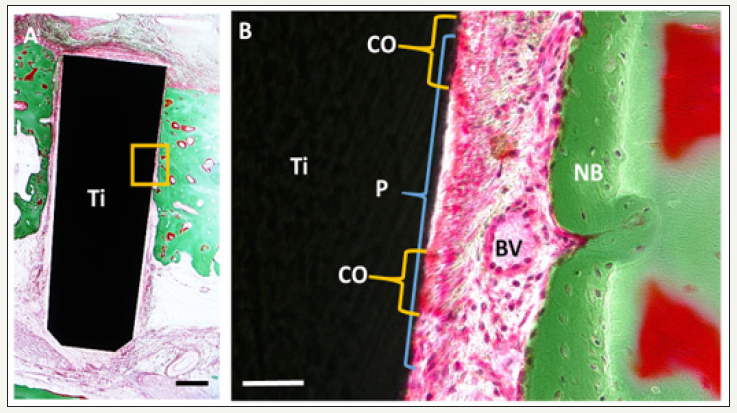
Recently, we successfully produced cementum and PDL on the implant surface using cell sheet engineering in canines [23]. We used PDL-derived cells as the cell source and produced a PDLderived cell sheet by culturing with osteo-inductive medium on the temperature-responsive culture dishes. As an implant material, we used the titanium implant, which was treated with an acid etching, blasting and a calcium phosphate (CaP) coating to strengthen cell attachment. The implants with enrolled canine PDL-derived cell sheets were transplanted to the canine mandibular bone. After an 11-week transplantation, histological observation showed cementum-like tissue and PDL-like tissue formation on the implant surface. The PDL-like fibrous tissue was perpendicularly oriented to the implant surface, which was similar to native PDL (Figure 1). Titanium implants were transplanted with PDL cell sheet in bone defects. Histological images of peri-implant tissues 11 month after implantation. The magnified image of boxed area in A is shown in B. Note that periodontal tissue with cementoid-like tissue was found on the titanium implant surface and PDL space, which was filled with PDL-like collagen fibers and blood vessels, between the implant and newly formed bone.
Figure 1:Formation of periodontal tissues around implant.
Ti: Titanium Implant; P: PDL-Like Tissue; CO: Cementoid-Like Tissue; BV: Blood Vessels; NB: New Bone. Figure 6A and B from Washio K et al. [23]. “In vivo periodontium formation around titanium implants using periodontal ligament cell sheet.” Tissue Engineering Part A 24: 1273-1282 2018 with permission of Mary Ann Liebert, Inc., New Rochell, NY, USA.
The reasons we obtained favorable results are as follows. First, we used cell sheet engineering, which allowed us to harvest PDL-derived cell sheets with the extracellular matrix including type I collagen, integrin beta I, and fibronectin [24]. Second, immobilization of the PDL-derived cell sheet on the modified surface of the titanium implant was effectively performed. We could confirm that the CaP-coated titanium implant accelerates cell adhesion to the titanium surface. The effect of CaP coating on titanium for periodontal formation was supported by a previous report [25]. Our in vivo study suggested that the cell sheet engineering and surface modifications to strengthen cell attachment of the implant were useful for the construction of an implant with periodontium, although improvements are still necessary in these procedures.
Cementum formation on implant as a future direction
As mentioned, a fully functional implant with ample periodontal tissues that can replace conventional dental implants has yet to be achieved. Cementum formation on an implant is necessary to achieve an implant with periodontium, because the cementum with the insertion of collagen bundles of PDL (also called “Sharpey’s fiber”) is the essential attachment structure of periodontium. However, it seems to be a challenging task to induce cementogenesis on the titanium surface artificially, because the differentiation mechanisms of cementoblasts, cementum forming cells, are unknown.
Many studies about cementoblast differentiation have been reported both in vitro and in vivo. Komaki et al. [26] have demonstrated that expression of CEMP-1, putative cementoblast marker, was diminished by osteoblastic differentiation induction (combination of ascorbic acid/ dexamethasone/ -glycerophosphate). Gauthier et al. [27] also demonstrated the same results and found enhancement of CEMP-1 by ascorbic acid treatment in PDL cells. These results suggested that the induction of osteoblast differentiation decreases the cementoblast marker expression and it is increased by the stimulation of ascorbic acid alone.
Additionally, in vivo experiments have been performed using genetically modified mouse models. Wnt is one of the signaling molecules thought to be involved in cementogenesis. Lim et al. [28] have found thin cementum, reduced bone mineral, and wide periodontal space, in mice with Wnt deletion specifically in osteoblasts and odontoblasts. In mice with stabilized Wnt signaling in osteoblasts and cementoblasts using the osteocalcin promoter, Kim et al. [29] demonstrated an increase in cementoblast differentiation and thickness of the cementum. Moreover, it has also been reported that cells from Hertwig’s epithelial root sheath and epithelial cell rests of Malassez, important regulator of cementogenesis and homeostasis, produced Wnt3a [30]. These data suggested the positive regulation of Wnt on cementogenesis and it is conceivable that Wnt signaling molecules, as well as ascorbic acid, could be an inducer of cementogenesis. To construct functional implants with periodontium, this cementum inducing molecules and signaling may be useful.
Future clinical application and conclusion
As described in the section “Cell sheet engineering,” cell sheet engineering is now safely applied to regenerative treatments in various fields. Furthermore, the efficacy and safety of the use of autologous cells and cell sheet engineering have been evaluated in two separate clinical studies for periodontal regeneration [22,31,32]. These studies strongly suggested the wide range of applicability of cell sheet engineering in periodontal regenerative treatment.
Furthermore, regenerative therapy using autologous cells is a safer method for clinical application in terms of immune rejection compared with the use of allogenic cells and artificial materials. However, there are still matters to be solved, such as contamination during the culture process. In Japan, government regulates cell therapy in order to ensure the safety and efficacy of treatments [33]. This mini review outlined the novel dental implant that can avoid some complications of conventional osteointegrated implants by utilizing the surrounding periodontal tissues, mimicking the natural structure of the tooth. Using cell sheet engineering, we provided a novel tissue engineering method for periodontal formation around implant. Although many unanswered questions remain in the methodology, this new concept can initiate fully or partially functional implant therapy. With further improvements in the procedures, it may be possible to establish a reliable implant system that more closely resembles the natural tooth in structure and function in the future.
Acknowledgement
This work was supported by the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program, in the Project for Developing Innovation Systems ‘‘Cell Sheet Tissue Engineering Center (CSTEC),’’ from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, MEXT/ JSPS KAKENHI Grant Number JP 26861687. Dental Corporation Tokushin-Kai group also supported this work financially.
Conflict of Interest
I.I. is an adviser of the Dental Corporation Tokushin-Kai group. The remaining authors have no conflicts of interest to declare..
References
- Branemark PI, Adell R, Breine U, Hansson BO, Lindstrom J, et al. (1969) Intra-osseous anchorage of dental prostheses. I. experimental studies. Scand J Plast Reconstr Surg 3(2): 81-100.
- Koldsland OC, Scheie AA, Aass AM (2010) Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol 81(2): 231-238.
- Derks J, Schaller D, Hakansson J, Wennstrom JL, Tomasi C, et al. (2016) Effectiveness of implant therapy analyzed in a swedish population: prevalence of peri-implantitis. J Dent Res 95(1): 43-49.
- Lindhe J, Meyle J (2008) Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol 35(8 Suppl): 282-285.
- Ericsson I (1995) Biology and pathology of peri-implant soft tissues. Quintessence Publishing Co, inc, USA.
- Giannobile WV (2010) Getting to the root of dental implant tissue engineering. J Clin Periodontol 37(8): 747-749.
- Buser D, Warrer K, Karring T (1990) Formation of a periodontal ligament around titanium implants. J Periodontol 61(9): 597-601.
- Choi BH (2000) Periodontal ligament formation around titanium implants using cultured periodontal ligament cells: a pilot study. Int J Oral Maxillofac Implants 15(2): 193-196.
- Jahangiri L, Hessamfar R, Ricci JL (2005) Partial generation of periodontal ligament on endosseous dental implants in dogs. Clin Oral Implants Res 16(4): 396-401.
- Parlar A, Bosshardt DD, Unsal B, Cetiner D, Haytac C, et al. (2005) New formation of periodontal tissues around titanium implants in a novel dentin chamber model. Clin Oral Implants Res 16(3): 259-267.
- Gault P, Black A, Romette JL, Fuente F, Schroeder K, et al. (2010) Tissue- engineered ligament: implant constructs for tooth replacement. J Clin Periodontol 37(8): 750-758.
- Lin Y, Gallucci GO, Buser D, Bosshardt D, Belser UC, et al. (2011) Bioengineered periodontal tissue formed on titanium dental implants. J Dent Res 90(2): 251-256.
- Oshima M, Inoue K, Nakajima K, Tachikawa T, Yamazaki H, et al. (2014) Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci Rep 4: 6044.
- Nakajima K, Oshima M, Yamamoto N, Tanaka C, Koitabashi R, et al. (2016) Development of a functional biohybrid implant formed from periodontal tissue utilizing bioengineering technology. Tissue Eng Part A 22(17-18): 1108-1115.
- Okano T, Yamada N, Sakai H, Sakurai Y (1993) A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res 27(10): 1243-1251.
- Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, et al. (2001) Thermo- responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng 7(4): 473-480.
- Sato M, Yamato M, Hamahashi K, Okano T, Mochida J (2014) Articular cartilage regeneration using cell sheet technology. Anat Rec 297(1): 36- 43.
- Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, et al. (2004) Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 351(12): 1187-1196.
- Miyagawa S, Domae K, Yoshikawa Y, Fukushima S, Nakamura T, et al. (2017) Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. J Am Heart Assoc 6(4): e003918.
- Kanzaki M, Takagi R, Kokubo M, Masayuki Y (2017) Bio-artificial pleura using an autologous dermal fibroblast sheet. NPJ Regen Med 2: 26.
- Arakelian L, Kanai N, Dua K, Durand M, Cattan P, et al. (2018) Esophageal tissue engineering: from bench to bedside. Ann N Acad Sci doi: 10.1111/ nyas.13951.
- Iwata T, Yamato M, Washio K, Yoshida T, Tsumanuma Y, et al. (2018) Periodontal regeneration with autologous periodontal ligament-derived cell sheets - A safety and efficacy study in ten patients. Regen Ther 9: 38-44.
- Washio K, Tsutsumi Y, Tsumanuma Y, Yano K, Srithanyarat SS, et al. (2018) In vivo periodontium formation around titanium implants using periodontal ligament cell sheet. Tissue Eng Part A 24(15-16): 1273- 1282.
- Hasegawa M, Yamato M, Kikuchi A, Okano T, Ishikawa I (2005) Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng 11(3-4): 469-478.
- Dan H, Vaquette C, Fisher AG, Hamlet SM, Xiao Y, et al. (2014) The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials 35(1): 113-122.
- Komaki M, Iwasaki K, Arzate H, Narayanan AS, Izumi Y, et al. (2012) Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J Cell Physiol 227(2): 649-657.
- Gauthier P, Yu Z, Tran QT, Bhatti FU, Zhu X, et al. (2017) Cementogenic genes in human periodontal ligament stem cells are downregulated in response to osteogenic stimulation while upregulated by vitamin C treatment. Cell Tissue Res 368(1): 79-92.
- Lim WH, Liu B, Cheng D, Williams BO, Mah SJ, et al. (2014) Wnt signaling regulates homeostasis of the periodontal ligament. J Periodontal Res 49(6): 751-759.
- Kim TH, Lee JY, Baek JA, Lee JC, Yang X, et al. (2011) Constitutive stabilization of ß-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem and Biophys Res Commun 412(4): 549-555.
- Nemoto E, Sakisaka Y, Tsuchiya M, Tamura M, Nakamura T, et al. (2016) Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J Periodontal Res 51(2): 164-174.
- Chen FM, Gao LN, Tian BM, Zhang XY, Zhang YJ, et al. (2016) Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther 7(33).
- Washio K, Iwata T, Mizutani M, Ando T, Yamato M, et al. (2010) Assessment of cell sheets derived from human periodontal ligament cells: a pre-clinical study. Cell Tissue Res 341(3): 397-404.
- Yoshida T, Washio K, Iwata T, Okano T, Ishikawa I (2012) Current status and future development of cell transplantation therapy for periodontal tissue regeneration. Int J Dent 307024.
© 2018 Isao Ishikawa. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






