- Submissions

Full Text
Innovation in Tissue Engineering & Regenerative Medicine
Cancerous Caricature, Fester, Transformation: Necrotizing Sialometaplasia
Anubha Bajaji*
Histopathologist in A.B. Diagnostics, India
*Corresponding author:Anubha Bajaji, Histopathologist in A.B. Diagnostics, New Delhi, India
Submission: August 01, 2018;Published: August 15, 2018

Volume1 Issue1 August 2018
Introduction
Necrotizing sialometaplasia, delineated initially in 1973 by Abrams et al. [1] is an infrequent, self limiting, variably ulcerated, benign, reactive, necrotising inflammatory mechanism prevailing upon minor salivary glands of the hard palate. Necrotising sialometaplasia exemplifies < 1% of the biopsied oral lesions [2]. The lesion is significant enough that it may be misconstrued for a malignancy and compel an extensive and irrelevant surgery. The phenomenon is categorized as a subsidiary “tumour -like” in the WHO classification of the salivary glands tumours.
Epidemiology
Necrotising sialometaplasia is exceptional. The lesions generally commence in males, who are preponderantly affected, at 50 years and in females at 36 years. A five to one prevalence is manifested in Caucasians over Afro Caribbeans [3]. Classically , it originates from the sero-mucinous minor salivary glands (80%) in the hard palate, but any location within the oral cavity can be incriminated , constituting of upper and lower lip, maxillary sinus, floor of the mouth, gingiva, cheek, tongue , retromolar area , oral mucosa, tonsillar fossa, major salivary glands, nasal cavity, larynx, soft palate , soft hard palate junction [3,4]. Major salivary gland constitutes 10% of the clinical scenarios. Necrotising sialometaplasia may be perceived at any location that consists of salivary gland components. A subacute variant has been detailed.
Clinical Presentation
Clinically, necrotising sialometaplasia exhibits a deep seated ulcer, albeit a smattering of lesions enunciate a non ulcerated lump or mass [4]. The condition is generally unilateral although bilateral and metachronous deformities are perceived in almost 12% of the subjects. The clinical picture elucidates a rapidly enlarging mass confined to the palate, which may or may not ulcerate. Initial manifestation may be a localized purulent discharge besides spasmodic, agonizing pain referred to the ear, eye or pharynx. Anaesthesia of the greater palatine nerve can be the initial symptom, besides a painless lump, manifested by the vasa nervorum in the predominantly vasculitic aetiology. Ulceration is usually superficial, although full thickness necrosis of the palate has been described. The lesions inevitably recover in 2 to 12 weeks (Figure 1).
Figure 1:Prominent features.

Intralesional steroids do not appear to ameliorate the lesion or the concordant anaesthesia. A sub acute variant of the disorder has been elucidated, in addition to the lesions that are frequently painful with lack of ulceration and the histopathology displays a sub acute inflammatory effusion, representing the extent of the disease.
Differential Diagnosis
Different diagnosis of the characteristic ulcer comprise of direct traumatic ulcer, major apthous ulcer, syphilis, tuberculosis, deep mycoses, agranulocytosis, neutropenia and nicorandil- induced oral ulceration. The disease emanates at a wide range of age (15 to 83 years) with a median at 46 years [5]. It is preponderant in males with a male to female ratio as 2-3:1 [6]. The malignancies specifically requiring discrimination are squamous cell carcinoma, low grade mucopidermoid carcinoma and oncocytic neoplasms. Exceptionally, extra salivary sites are implicated such as the nose, nasopharynx, trachea, larynx and lung, where the ulcerated swelling may be referred to as adenometaplasia. Analogous dermal lesions are designated as syringometaplasia. Traumatic injury to the breast evokes congruous histopathological alterations.
Aetiopathogenesis
Though the aetiopathogenesis of necrotising sialometaplasia is obscure, the evolution of necrotising ulcers in the salivary glands is preceded by an ischemic situation. Sickle cell disease (and crisis) with infarction is an essential aspect of the disorder. Possible mediators in the evolution of the disease are direct trauma, administration of local anaesthesia, ill fitting dentures, alcohol, smoking, cocaine use, radiation, surgical procedures, upper respiratory infection and chronic vomiting [6]. Beurger’s disease and Raynauds’s phenomenon are established vasculopathies which induce ischemia. Necrotising sialometaplasia of the palate may inconsistently be portrayed as an ulcerative or necrotising phase of leukokeratosis nicotina palate. Ischemia may also be precipitated by smoking and alcohol intake along with vascular impairment caused by trauma, hot food, intubation, fellatio, bronchoscopy, local anaesthetic injection and recurrent vomiting. Inclusion of a vasoconstrictor to local anaesthetic solution, local radiotherapy, cocaine use, pressure from local space occupying lesion and surgery are also incriminated. Pregnancy induces aberrant lesions. Oncogenic combinations are specifically with Warthin‘s tumour, Abrisokov’s tumour, carcinoma of the lip, rapidly growing mesenchymal malignancy and salivary gland tumours. Acute on chronic sinusitis and allergy are implicated besides previous upper respiratory tract contamination. Ischemic events in the preceding clinical scenarios are probably due to immune complex disease aetiologically similar to erythema multiforme or benign trigeminal sensory neuropathy (Figure 2).
Figure 2:Specific aspects.

Histopathology
Abrams et al. [1] propounded the following microscopic pattern: Necrosis of acinar cells of the seromucinous glands, squamous metaplasia of the salivary duct and acinar epithelium, pseudo-epitheliomatous hyperplasia of the epithelium lining the gland, mucous polling, a granulomatous inflammatory response in and around the glands, benign nuclear morphology on histology although normal mitosis can emerge. Brannon et al described the prominence of coagulative necrosis of acini in early squamous metaplasia and reactive fibrosis in the subsequent lesions. Anneroth & Hansen [2] propose five histological subdivisions in the progression of necrotising sialometaplasia: Infarction, Sequestration, Ulceration Repair and Healing [7]. Determined by the magnitude and severity of the destruction, the phases may extend and overlap. Extensive infarction results in the segregation of the necrotic acini which contributes to the formation of an ulcer (Figure 3). Sequestration with ulceration does not manifest, when the infarction is limited. The typical squamous metaplasia of the duct components may be mistaken for a squamous cell carcinoma. Specific metaplasia in the viable salivary gland residuum may be misinterpreted as a mucoepidermoid carcinoma. The histological alterations that simulate a carcinoma are caused by the latent reparative alterations but are not attributable to the constitutional features of the condition. However, the possibility of a concurrent malignancy should be considered, thus requiring a biopsy and recognizing the disease process [8]. Histological benchmarks to demarcate necrotising sialometaplasia from malignant transformation are
A. Preserved general lobular morphology.
B. Bland expression of the squamous islands or nests with no cytological confirmation of malignancy.
C. Absence of residual ductal lumina in a particular cell nest with infrequent reactive atypia. The characteristic lobular display and the existence of intraepithelial inflammation in the squamous nests assist in clinching the diagnosis.
D. Necrotising sialometaplasia should be distinguished from traumatic, inflammatory and infectious ulcerative disorders such as traumatic ulcer, major apthae, tuberculosis, tertiary syphilis or deep seated fungal disease, patients on chemotherapy and immunosuppressive treatment, underlying malignancies, Auto Immune Deficiency Syndrome (AIDS) etc.
Figure 3:Necrotising Sialometaplasia with pseudoepitheliomatous hyperplasia.
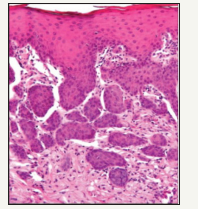
Sub acute necrotising sialo-adenitis should be considered as a part of the spectrum of necrotising sialometaplasia, designated as a non specific acute inflammatory condition of obscure origin with a histological demarcation due to focal acute necrosis (secondary to inflammation) and atrophy of duct cells without duct metaplasia, pseudo epitheliomatous hyperplasia or fibrosis [9]. Clinically, it expresses as a non ulcerated, acutely painful, erythematous nodular lesion located on the palate, frequently detected in the cohabiting youth. Viral infections or allergy are the hypothetical aetiological agents (Figure 4). The prominent dissimilarities from necrotising sialometaplasia are the smaller size of the lesion, paucity of ulceration and absence of squamous metaplasia. Generally necrotising sialometaplasia does not warrant any therapeutic intervention. The ulcers recover by secondary intention within 4 to 10 weeks (average 5.2 weeks). Full thickness palatal ulcers, contiguous with the nasal cavity, may recover entirely within 6 months [10]. Subsequent to the initial delineation by Abrams et al. [1] in 1973, the histological characters and differential diagnosis of necrotising metaplasia have been reviewed by numerous authorities. Histopathology describes the ischemic lobular necrosis of seromucinous glands with preserved, compact lobular architecture despite the coagulative necrosis of the mucinous acini. Pale acinar outlines often persist, but the nuclei are hypochromatic or absent (Figure 5).
Figure 4:Coagulative necrosis with metaplasia and hyperplasia of duct and acinar epithelium.
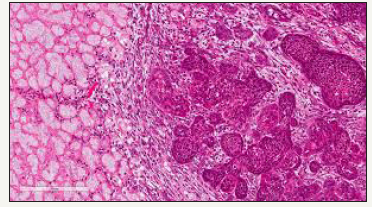
Figure 5:Sqaumous islands in a background of fibrogranulation tissue- necrotising sialometaplasia.
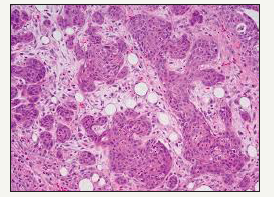
Mucin extravasation into the surrounding tissues stimulates an inflammatory response predominated by histiocytes. Granulation tissue within the necrotic lobules coexisting with the inflammatory element is often minimal, but is generally conspicuous in the adjacent tissues. Even though squamous metaplasia of ducts and acini can be a confounding aspect of malignancy, the metaplastic cells exhibit benign nuclear morphology with minimal pleomorphism or hyperchromatism and few mitotic figures. Nests of squamous epithelium exhibit a typically smooth periphery besides nests with a random, irregular outline. Pseudo epitheliomatous hyperplasia is elucidated in the adjacent, hyperplastic epithelium with substantial, elongated and intricate rete formations accompanying extensive duct metaplasia. The features resemble epithelial malignancy and an ambiguous diagnosis may account for disproportionate and radical ablative surgery. It can be baffling to differentiate necrotising metaplasia from squamous cell carcioma, low grade mucoepidermoid carcinoma and oncocytic tumours. Histological demarcation is dependent upon the duration of the lesion submitted for biopsy. Coagulative necrosis is prominent in the early lesions (Figure 6).
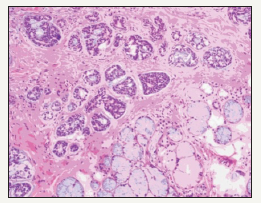
Figure 6:Squamous Islands with bland cytology-sialometaplasia.

Management
Generally, the disorder is treated by preliminary observation and analysis until the lesion advances. Necrotizing sialometaplasia may arise de novo, may be secondary to a traumatic surgical procedure or in concordance with a benign or malignant lesion. Necrotising sialometaplasia when encountered necessitates an extended follow up until recovery. The histological depiction and the diverse clinical scenarios in which necrotising sialometaplasia can be identified requires constant review, so that a microscopic exaggeration is avoided with subsequent disproportionate therapy for what is an essentially benign, reactive infirmity. The prognosis is excellent, once the diagnosis is established. There are no specific preventive strategies (Figure 7).
Figure 7:Necrotising Sialometaplasia with coagulative necrosis.
References
- Abrams AM, Melrose RJ, Howelll FV (1973) Necrotising sialometaplasia. A disease simulating maliganancy. Cancer 32(1): 130-135.
- Anneroth G, Hansen LS (1982) Necrotising sialometaplasia: The relationship of it’s pathogenesis to it’s clinical characteristics. Int J Oral Surg 11(5): 283-291.
- Mesa MI, Gertler RS, Schneider LC (1984) Necrotising sialometaplasia: Frequency of histological misdiagnosis. Oral Surg Oral Med Oral Pathol 57(1): 71-73.
- Van der Wal JE, Van der Wal I (1990) Necrotising sialometaplasia: Report of 12 new cases. Br J Oral Maxillofac Surg 28(5): 326-328.
- Brannon RB, Fowler CB, Hartman KS (1991) Necrotising sialometaplasia: A clinicopathologic study of 69 cases and review of the literature. Oral Surg Oral Med Oral Pathol 72(3): 317-325.
- Carlson DL (2009) Necrotising sialometaplasia: A practical approach to the diagnosis. Archives of Pathol and Lab Medicine 133(5): 692-698.
- Rye LA, Calhoun NR, Sedman RS (1980) Necrotising sialometaplasia in a patient with Beurger’s disease and Raynaud’s phenomenon. Oral Surg Oral Med Oral Pathol 49(3): 233-236.
- Lee DJ, Ahn HK, Koh ES, Rho YS, Chu HR (2009) Necrotising sialometaplasia accompanied by adenoid cystic carcinoma on the soft palate. The J of Clin and Exp Otorhinolaryngology 2(1): 48-51.
- Fowler CB, Brannon RB (2000) Subacute necrotising sialadenitis: report of seven cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89(5): 600-609.
- Daudia A, Murty GE (2002) First case of full thickness palatal necrotising sialometaplasia. The Journal of Laryngology and Otology 116(3): 219- 220.
© 2018 Anubha Bajaji. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






