- Submissions

Full Text
Intervention in Obesity & Diabetes
Atypical Endometrial Cancer, Obesity, Diabetes and Hyperandrogenism
Susana Leiderman1*, Doris Rodríguez Vidal1, Eugenia Lamas Majek1, María José Ojué1, Julieta Giarmana1, Sebastián Alessandría2, Carla Britos2, Lucía Cardinal3, Patricia Maidana4, Javier Ortiz5 and Gladys Fernández1
1Gynecologic Endocrinology Section, Gynecology Division, Hospital de Clínicas José de San Martín, Buenos Aires University, Argentina
2Gynecologic Oncology Section, Gynaecology Division, Hospital de Clínicas José de San Martín, Buenos Aires University, Argentina
3Gynecologic Pathology Division, Pathology Department, Hospital de Clínicas José de San Martín, Buenos Aires University, Argentina
4Rossi Diagnostic Center Laboratory, City of Buenos Aires, Argentina
5Chief of Gynecology Division, Hospital de Clínicas José de San Martín, Buenos Aires University, Argentina
*Corresponding author:Susana Leiderman, Gynecologic Endocrinology Section, Gynecology Division, Hospital de Clínicas José de San Martín, Buenos Aires University, Argentina
Submission:April 16, 2024;Published: May 07, 2024

ISSN 2578-0263Volume6 Issue4
Abstract
Herein, we report a case of a 50-year-old female patient with advanced endometrioid carcinoma and several associated metabolic and endocrine disturbances. The patient was on hormonal contraception therapy with progestins and was referred because of clinical hyperandrogenism. An ultrasound examination revealed a thickened endometrium and the patient was sent for hysteroscopy, which reported endometrioid adenocarcinoma. In addition to known hypertension, dyslipidemia and type 2 diabetes, metabolic and hormonal laboratory test results showed hyperinsulinemia and hyperandrogenism. Bilateral oophorectomy was performed. The uterus could not be removed due to the advanced stage of disease. The ovaries revealed metastasis from endometrioid carcinoma on a background of bilateral stromal hyperthichotic. In this article, we discuss the role of hyperandrogenism and hyperinsulinemia in the development of this advanced cancer.
Keywords:Endometrial cancer; Hyperinsulinemia; Hyperandrogenism; Obesity; Oligomenorrhea
Introduction
Endometrial cancer is the second most common gynecologic cancer in Argentina, accounting for 6% of all cancers in women [1]. It has a relatively good overall prognosis and is classified as endometrioid and non-endometrioid. The former is the most common subtype and is characterized for being diagnosed at an early stage, being hormone-dependent and having a more favorable clinical course. Risk factors include obesity, hypertension, hyperinsulinemia, chronic exposure to unopposed estrogen (chronic anovulation, polycystic ovary syndrome, late menopause, etc.), and environmental pollutants; conversely, physical exercise and long-term use of Combined Oral Contraceptives (COCs) are associated with a lower risk of endometrial cancer [1,2]. The incidence and mortality rate of this disease is increasing as a result of the growing prevalence of obesity, a known risk factor for the most common type of endometrial cancer [3].
Case Presentation
A fifty-year-old patient was referred to the Menopause and Gynecologic Endocrinology Section of the Gynecology Division at Hospital de Clínicas José de San Martín in April 2023 due to clinical hyperandrogenism with suspected diagnosis of perimenopausal Polycystic Ovary Syndrome (PCOS). The patient was non-smoker, reported no allergies and had a sedentary lifestyle.
Medical history
The patient had myasthenia gravis caused by thymoma at the age of 16. She underwent thymectomy complicated by hemothorax that required blood transfusion. She was then treated with 60mg daily of methyl prednisone for 9 years, which was associated with a weight gain of 40kg, hypertension, amenorrhea and cataract development. She subsequently received azathioprine and pyridostigmine. Currently, she is only on treatment with pyridostigmine. She has a history of blood hypertension since the age of 20, type 2 diabetes diagnosed at the age of 36, cirrhosis secondary to post-transfusion hepatitis C and dyslipidemia at the age of 49. On admission, she was receiving pyridostigmine 60mg/ day, enalapril/hydrochlorothiazide 10/25mg/day, amlodipine 5mg/day, metformin 1000mg/day, rosuvastatin 10mg/day and 0.075mg of desogestrel.
Gynecologic history
The patient experienced her menarche at 11 years of age and had irregular menstrual cycles until the age of 16. She started with secondary amenorrhea at this age, concurrent with the intake of high doses of glucocorticoids until the age of 24. After glucocorticoid discontinuation, menstrual cycles resumed, with oligomenorrhea and periods of abnormal uterine bleeding. At the age of 35, due to persistent oligomenorrhea associated with clinical signs of hyperandrogenism (hirsutism, acne), she was diagnosed with PCOS and received COCs consisting of 30 micrograms of ethinylestradiol and 3 milligrams of drospirenone until the age of 49 years, when treatment was switched to Desogestrel (DSG) 75 micrograms until the time of consultation. The patient never got pregnant, on her own accord.
Family history
Mother with hypertension and type 2 diabetes mellitus. Maternal aunt and grandmother with breast cancer.
Physical examination
On physical examination, she had a body weight of 108.600kg,
height of 1.58m, Body Mass Index (BMI) of 43.5kg/m2, blood
pressure of 140/70mmHg and heart rate of 76bpm. Her Body
Composition (OMRON HBF-514C scale) was body fat 49.6% (Normal
Value [NV] for a 40-59-year-old woman: 23-33.9%), muscle mass
32.1% (NV for a 40-59-year-old woman: 24.1-30.1%) and visceral
fat 13 (NV9). Waist circumference was 132.3cm. Normal thyroid,
with no nodules or irregularities detected on palpation. Patient
with morbid obesity. Presence of acanthosis nigricans in the neck.
Central obesity with presence of pearly white striae over the whole
body, a hump on upper back and florid complexion. Normal breast
examination. At the time of visit, the patient was on DSG and
reported progressive increase in facial and body hair associated
with acne. She epilated her facial and body hair every other day and
reported thicker and more pigmented hair (Figure 1). The following
studies were provided by the patient:
a. Mammography (20-OCT-22): normal breasts, BI-RADS 2.
b. Transvaginal ultrasound (9-MAR-23): regular uterus
with heterogeneous, thickened endometrium (22-mm thick).
Ovaries of normal sonographic characteristics. Douglas’ pouch
clear.
Figure 1:Central obesity with presence of pearly white striae over the whole body.
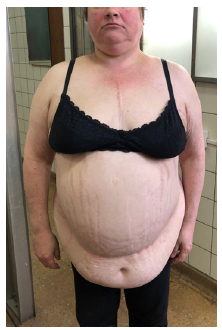
Figure 2:MRI with large mass in the right ovary with projection into the abdominal cavity in the ipsilateral iliac fossa.
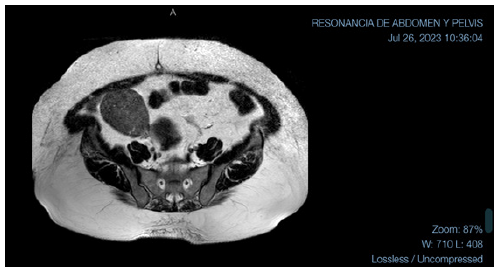
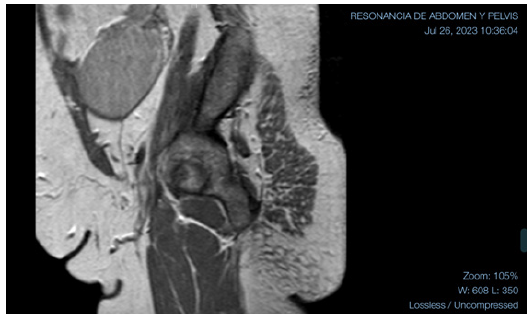
The patient was sent for hysteroscopy for endometrial biopsy and serum androgen measurement was ordered. The hysteroscopy was performed on 10-MAY-23. The endometrial biopsy pathology reported “Moderately Differentiated Endometrioid Adenocarcinoma (G2).” The patient was referred to oncologist, where Magnetic Resonance Imaging (MRI) was ordered for staging purposes (26-JUL-23). The MRI showed uterus in anteversion and anteflexion, left ovary with some cystic lesions of homogeneous content and, in the right ovary, an extensive solid mass, 82-mm in diameter, homogeneously enhancing after contrast administration, with projection into the abdominal cavity in the ipsilateral iliac fossa. Even if this lesion could be consistent with adnexal disease, an extensive subserosal myoma could not be ruled out. The scan showed no enlarged endopelvic or inguinal lymph nodes. Further evaluation by transvaginal ultrasound was suggested (Figure 2 & 3).
Figure 3:MRI with extensive solid mass, 82-mm in diameter, homogeneously enhancing after contrast administration.
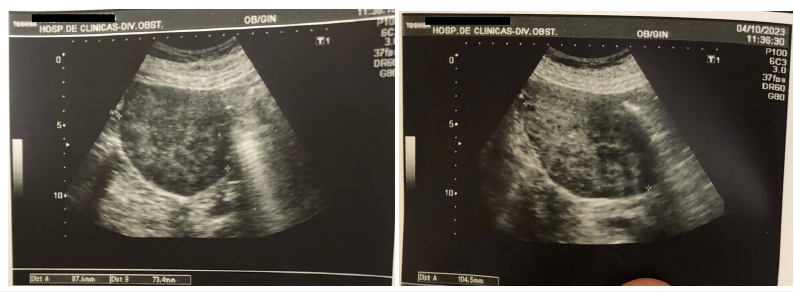
The oncologist ordered tumor marker testing, which was negative, and transvaginal Doppler ultrasound, which was performed on 4-OCT-23. The ultrasound scan showed a 15- mm endometrium. Right adnexal region with cystic lesion with wall thickening in an area that exhibited increased vascularity. The lesion measured 21 x 14mm. The left ovary was not visible. Douglas’ pouch clear. In close contact with the fundus of uterus, there was a well-defined, hypoechoic, heterogeneous nodular lesion of solid appearance measuring 104 x 73 x 87mm and with poor peripheral signal on power angio. The patient provided the results of the laboratory tests performed on 9-OCT-23 (Table 1) while on treatment with DSG: total cholesterol 106mg/dL, High- Density Lipoprotein (HDL) cholesterol 71mg/dL, triglycerides (TG) 75mg/dL blood glucose 104mg/dL, insulin 65.6IU/ml, nocturnal salivary cortisol (11 p.m.) 0.17g/dL (NV<0.43g/ dL), estradiol (E2) 37.6pg/mL , Estrone (E1) 144pg/mL, Follicle- Stimulating Hormone (FSH) 8.5mIU/ml, Luteinizing Hormone (LH) 5.3mIU/mL, Total Testosterone (TT) 0.73ng/mL, Free Testosterone (FT) 8.04pg/mL, delta-4-androstenedione (D4A) 2.02ng/mL, 17-hydroxyprogesterone (17-OHP) 4.23ng/mL (Figure 4). On 29-NOV-2023, the patient underwent exploratory laparotomy with peritoneal lavage, bilateral adnexectomy, omentectomy and biopsy sampling. The study showed multiple implants <1cm in the parietal peritoneum. Implants were found in the liver, the serosa of the small intestine, colon and mesocolon. Samples were collected for pathology examination. The momentum contained multiple nodular masses. The right ad nexus was enlarged at the expense of an approximately 8-cm cystic mass with smooth surface and fluid content. Bilateral adnexectomy was performed. The intraoperative pathology of the right ad nexus and implants showed infiltration by endometrioid carcinoma. Fixed uterus with infiltrating implants in the vesicouterine junction and left parametrium. Given the impossibility of respectability, a decision was made not to perform hysterectomy.
Table 1:Pre-surgical hormone levels (on DSG) and 48 hours after surgery. Abbreviations: 17-OHP: 17-Hydroxyprogesterone, CLIA: Chemiluminescent Immunoassay, D4A: Delta-4-Androstenedione, DHEAS: Dehydroepiandrosterone Sulfate, E1: Estrone, E2: Estradiol, ECLIA: Electrochemiluminescence Immunoassay, FSH: Follicle-Stimulating Hormone, FT: Free Testosterone, LH: Luteinizing Hormone, NA: Not Applicable, NV: Normal Value, RIA: Radioimmunoassay, TT: Total Testosterone.

Figure 4:Ultrasound with hypoechoic, heterogeneous nodular lesion of solid appearance measuring 104 x 73 x 87mm and with poor peripheral signal on power angio.
DSG was discontinued on the day of surgery and blood tests
were performed 48 hours after surgery (1-DEC-23) (Table 1). The
pathology report of the surgical specimen revealed:
i. Right and left adnexa: Bilateral Ovarian Metastasis From
Endometrioid Carcinoma (G2) 15 X 1cm and 6 x 3cm in size,
respectively.
ii. Bilateral Stromal Hyperthichotic. Tubes with no
abnormalities.
iii. Lymph vascular Invasion: Present
iv. Implants and momentum: Infiltration by Carcinoma
v. Peritoneal lavage; cytology: Positive for Neoplastic Cells.
The patient is currently being treated by the gynecologic oncology section with paclitaxel and carboplatin (Figure 5).
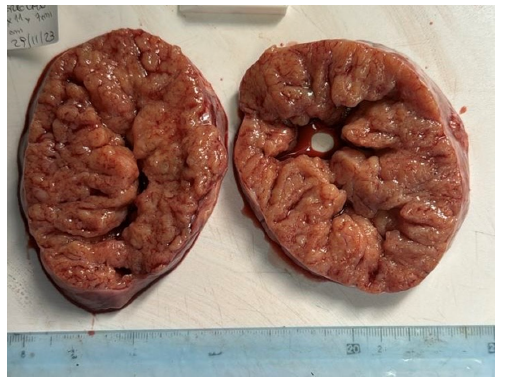
Figure 5:A solid friable golden-brown right ovarian mass (15cm).
Discussion
This case history apparently began with the alteration of the patient’s menstrual cycles in adolescence, when she already experienced oligomenorrhea with a normal BMI of 24.8kg/m2, despite a gynecological age of 5 years. With the development of a thymoma and treatment with high doses of glucocorticoids, her BMI increased to 43.5Kg/m2 and the patient become amenorrhea. Upon discontinuation of glucocorticoids, oligomenorrhea resumed, which was associated with clinical hyperandrogenism (hirsutism, acne). Therefore, she was diagnosed with PCOS and was prescribed COCs. She received COCs for 14 years and then was switched to progestins alone. The risk factors for hormonedependent endometrial cancer include the widely known triad of hypertension, obesity and diabetes, which was already present in this patient at the age of 36 years [2]. The use of COCs is known to have a protective effect against endometrial cancer [4-6]. This is due to the suppressive action of COCs on LH production, resulting in decreased steroidogenic activity with lower production of ovarian androgens, which will be subsequently converted to estrogen. In addition, the estrogenic component of COCs stimulates the production of Sex-Hormone-Binding Globulin (SHBG), with a consequent decrease in free estrogens [7].
However, despite the long-term use of COCs, our patient developed endometrial cancer. Even if no hormone measurements prior to the use of contraceptives are available, the correction of menstrual cycles was considered to be sufficient. In addition, the patient’s treatment for diabetes was not associated with any changes in her lifestyle that might lead to weight loss. It is known that obesity increases the risk of endometrial cancer due to multiple mechanisms [8-11]. On the one hand, it has been documented that (mainly visceral) obesity increases insulin resistance with compensatory hyperinsulinemia leading to increased signaling of the insulin-IGF-1 axis [12,13]. On the other hand, obesity is associated with an increased aromatase activity in the uterus, converting androgens to estrogens; in addition, decreased SHBG contributes to an increase in free steroids. It is well-known that obesity is associated with an increase in inflammatory cytokines and a decrease in adiponectin, an increase in oxidative stress and advanced glycation end products, and an abnormal immune response, with all these factors being closely associated with the increased risk of cancers seen in obese individuals [14-16].
Finally, Asaka et al. [17] point out that SIRT1 (Sirtuin-1) may be downregulated in normal endometrial glandular cells of obese women, and that downregulation of SIRT1 may be involved in facilitating endometrial carcinogenesis [17]. Thus, in this case, it is not the protective effect of contraceptives but the risk of longterm hyperinsulinemia what seems to prevail. The lack of hormone measurements performed while the patient was on COCs does not allow us to ensure suppression of her ovarian hyperthichotic. However, it was evident that the use of progestins alone did not inhibit androgen production by the nests of theca cells present in hyperthichotic. Could the aggressive behavior of this cancer be attributed to hyperinsulinemia?
Conclusion
Despite regularization of menstrual cycles with COCs in patients with oligomenorrhea and clinical signs of hyperandrogenism, in those who also have diabetes and obesity we should not overlook the harmful role of hyperinsulinemia. Accordingly, in addition to hormone measurements, metabolic screening should be performed with the aim of identifying patients at increased risk of cancer in order to offer a multidisciplinary approach for better management of these women.
References
- Odetto D, Puga M, Rey Valzacchi G, Saadi JM, Zamora LB, et al. (2023) High-risk endometrial carcinoma in early stages: Hospital Italiano de Buenos Aires, oncological results. Rev Fac Cien Med Univ Nac Cordoba 80(4): 352-366.
- Oaknin A, Bosse T, Creutzberg CL, Giornelli G, Harter P, et al. (2022) Endometrial cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 33(9): 860-877.
- McDonald M, Bender D (2019) Endometrial cancer: Obesity, genetics, and targeted agents. Obstet Gynecol Clin North Am 46(1): 89-105.
- Cibula D, Gompel A, Mueck AO, Vecchia CL, Hannaford PC, et al. (2010) Hormonal contraception and risk of cancer. Hum Reprod Update 16(6): 631-650.
- Iversen L, Fielding S, Lidegaard Ø, Hannaford P (2020) Contemporary hormonal contraception and risk of endometrial cancer in women younger than age 50: A retrospective cohort study of Danish women. Contraception 102(3): 152-158.
- Grimbizis G, Tarlatzis B (2010) The use of hormonal contraception and its protective role against endometrial and ovarian cancer. Best Pract Res Clin Obstet Gynaecol 24(1): 29-38.
- ESHRE Capri Workshop Group (2001) Ovarian and endometrial function during hormonal contraception. Hum Reprod 16(7): 1527-1535.
- Bifulco M, Pisanti S (2013) Adiponcosis: A new term to name the obesity and cancer link. J Clin Endocrinol Metab 98(12): 4664-4665.
- Crosbie E, Kitson S, McAlpine J, Mukhopadhyay A, Powell ME, et al. (2022) Endometrial cancer. Lancet 399(10333): 1412-1428.
- Pati S, Irfan W, Jameel A, Ahmed S, Shahid R (2023) Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers 15(2): 485.
- Harborg S, Kjærgaard K, Wernich Thomsen R, Borgquistet S, Fenton DC, et al. (2024) New horizons: epidemiology of obesity, diabetes mellitus, and cancer prognosis. J Clin Endocrinol Metab 109(4): 924-935.
- Renehan A, Jan Frystyk J, Flyvbjerg A (2006) Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab 17(8): 328-336.
- Zhong W, Wang X, Wang Y, Sun G, Zhang J, et al. (2023) Obesity and endocrine-related cancer: The important role of IGF-1. Front Endocrinol 14: 1093257.
- Brown K, Scherer P (2023) Update on adipose tissue and cancer. Endocr Rev 44(6): 961-974.
- Vella V, Lappano R, Bonavita E, Maggiolini M, Clarke RB, et al. (2023) Insulin/IGF axis and the receptor for advanced glycation end products: role in meta-inflammation and potential in cancer therapy. Endocr Rev 44(4): 693-723.
- Rathmell J (2021) Obesity, immunity, and cancer. N Engl J Med 384(12): 1160-1162.
- Asaka R, Miyamoto T, Yamada Y, Ando H, Mvunta DH, et al. (2015) Sirtuin 1 promotes the growth and cisplatin resistance of endometrial carcinoma cells: A novel therapeutic target. Lab Invest 95(12): 1363-1373.
© 2024 Susana Leiderman. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






