- Submissions

Full Text
Intervention in Obesity & Diabetes
Adiponectin; A Friend or Foe in Type 2 Diabetes Mellitus? A Review
Saima Sharif*, Saima Nasir, Shagufta Naz, Tasnim Farasat and Farkhanda Manzoor
Department of Zoology, Faculty of Science and Technology, Lahore College for Women University Lahore, Pakistan
*Corresponding author:Saima Sharif, Department of Zoology, Faculty of Science and Technology, Lahore College for Women University Lahore, Jail Road, Lahore, 54000, Pakistan
Submission:September 16, 2022;Published: October 19, 2022

ISSN 2578-0263Volume6 Issue1
Abstract
The epidemic spread of diabetes is a modern-day health crisis. Treating and preventing diabetes has been identified as a major public health issue across the world. Type 2 Diabetes Mellitus (T2DM) is the most prevalent forms of the diabetes, and it is caused by a combination of environmental, behavioural, and genetic risk factors. There is a threefold increased risk of type 2 diabetes in those with a family history of diabetes mellitus compared to the unrelated individuals. The Adiponectin (AdipoQ) gene is only one of several genes that have a role in the progression of diabetes. Adiponectin a 30kDa protein hormone derived from an adipocyte, is encoded by the AdipoQ gene carrying a susceptibility locus for type 2 diabetes mellitus located on chromosome 3q27 which regulates adiponectin the most plentiful Adipokine in human plasma. The adiponectin has anti-inflammatory, antiatherogenic and insulin-sensitising properties that plays a censorious role in the progression of insulin resistance and type 2 diabetes mellitus. Plasma and serum levels of adiponectin (AdipoQ) are inversely correlated with type 2 diabetes mellitus. Single nucleotide polymorphism (SNPs) of the AdipoQ gene have been strongly associated with the pathogenicity of type 2 diabetes mellitus. However, there is still some debate about the findings, and there are clear racial and geographical differences. Reviewing what is known about Adiponectin and the Adiponectin (AdipoQ) Gene, with a focus on its function in the physiology and pathophysiology of type 2 diabetes mellitus, is the goal of this article. Adiponectin was found to play a significant pathological role in the progression of type 2 diabetes mellitus.
Keywords:Adiponectin (AdipoQ); Type 2 diabetes mellitus; Pathogenesis; Insulin sensitivity; Polymorphism; Expression
Introduction
The term “Diabetes Mellitus” refers to a wide range of metabolic illnesses characterized by persistent hyperglycemia. The condition can be caused by either a malfunction in insulin production or a variation in degree of insulin resistance [1]. Diabetic complications are more common, and their prevalence is on the rise. Because of factors such as urbanization, increasing age, physical inactivity, obesity, and sedentary lifestyles and bad eating habits, diabetes is on the rise across the world [2]. 8.4% of the world’s deaths are caused by diabetes mellitus [3]. In 2021, the global prevalence of diabetes in 20-79-year-olds was expected to be 536.6 million people which is 10.5%, increasing to 783.2 million approximately 12.2% in 2045. Women were more likely to be diagnosed with diabetes than males; individuals aged 75-79 had the highest frequency. Prevalence of diabetes in 2021 was expected to be higher in urban regions which is 12.1% than rural regions approximately 8.3%, and in high-income nations almost 11.1% compared to some low-income nations about 5.5%. Middle-income nations are predicted to see the highest rise in diabetes prevalence between 2021 and 2045 almost 21.1% compared to high- and low-income countries, 12.2 % and 11.9 % respectively. According to the World Health Organization In 2021, global diabetes-related health costs were estimated at 966 billion USD and are expected to rise to 1,054 billion USD till 2045 [4].
Type 2 Diabetes Mellitus
T2DM continues to be considered one of the most significant health challenges faced by people all over the world [5]. 1 in 9 fatalities in those aged 20-79 is associated with type 2 diabetes [6]. Rapid progress has been made in our understanding of type 2 diabetes during the past several decades. It is caused by insulin resistance in liver, skeletal muscle, and adipose tissue, which in turn is caused due to impairment in insulin secretion by pancreatic cells [7].
Pathogenesis of Type 2 Diabetes Mellitus
Despite the fact that one’s way of life has a part in the development of type 2 diabetes, hereditary factors enhance one’s vulnerability to develop the disease [8]. Insulin resistance and changes in insulin production and secretion are two mechanisms through which risk alleles raise the risk of developing type 2 diabetes [9]. Patients with type II, NIDDM or Non-Insulin dependent Diabetes Mellitus have an increased overall requirement for insulin necessary for physiological glucose absorption. At first, pancreatic cells respond to increased demand by producing more insulin, but as the demand continues to rise, the pancreatic cells undergo changes, and insulin production begins to decline [10]. The failure of islet beta cells in obesity-related type 2 diabetes is a consequence of the body’s inability to maintain sufficient insulin production to account for insulin resistance, which suggests that the failure of islet beta cells as a casualty and a downstream effect of insulin resistance [11]. Nonetheless, there is growing evidence both from preclinical and clinical research to support an alternative interpretation, at least in clusters of people at risk of T2DM, overreaction of pancreatic β-cells to an unfavourable environment (e.g., influenced from western culture) promotes hyperinsulinemia, being the root cause of type 2 diabetes, obesity, insulin resistance, and beta-cell failure [12,13]. It is believed that insulin resistance during Type-2 diabetes is induced by both hereditary and environmental factors. Disease susceptibility may vary among populations due to genetic factors. Some people have a higher predisposition to acquire type 2 diabetes due to inherited risk factors [14]. Over 70% risk of type 2 diabetes has been linked to inherited factors, and research has found that various subgroups of people may be affected by a distinct set of genes or gene combinations [15]. Genetic studies of AdipoQ gene reveal that adiponectin plays an important function in the aetiology of type 2 diabetes and may also play a part in determining one’s vulnerability to insulin resistance.
Adiponectin (AdipoQ)
Adiponectin, a secretory protein of 30kDa, was discovered in 1995. In humans widely secreted by 3T3 L1 adipocytes as well as observed in large plasma levels in mice [16]. The white adipose tissues synthesize this protein called adiponectin, which has 224 amino acids [17]. ACRP30, apM1, GBP28 and AdipoQ are all names for adiponectin [18]. AdipoQ gene is responsible for encoding adiponectin [19]. One of the most important things that goes into the synthesis of adiponectin is the level of expression of the AdipoQ gene [20]. The adiponectin gene, also known as AdipoQ, found in the 3q27 region of the chromosome and takes up around 17kb of the deoxyribonucleic acid in its whole. It has been determined that this particular area is a susceptibility locus for type 2 diabetes Mellitus and metabolic syndrome [21]. The AdipoQ gene is made up of a total of five segments: two introns and three exons [22]. Adiponectin unique contribution to enhancing insulin sensitivity, fatty acid betaoxidation and modifying islet β-cell dysfunction, is thought to play a significant part in the pathogenesis of type 2 diabetes mellitus [21]. Within a range of 5 to 30μg/mL, adiponectin is considered to be at a healthy concentration in the human bloodstream [23].
Adiponectin Structure
Adiponectin’s high-resolution structure was determined by Scherer & Shapiro [24]. A signal area is located at the NH2 terminus of the adiponectin protein, a variable region that is distinct to each species, a collagenous domain, and a globular domain are located somewhere at COOH terminus of the adiponectin (AdipoQ) protein, respectively [25]. Adiponectin can exist in the forms of a trimer (with a molecular weight of about 90kDa), a hexamer (with an average molecular weight 180kDa; this is a sort of LMW form), a multimer or High molecular form weighing almost 400kDa). Protein breakdown products (which include a spherical endspin domain) are detected inside the body, despite the fact that the longitudinal form is not often present under normal settings due to its high thermodynamic destabilisation [26]. Globular adiponectin, a globular C1q domain of AdipoQ produced from full-length protein via proteolytic cleavage, is also physiologically active [27]. Due to differences in affinity for receptors and other cellular targets, the different forms have varying biological effects [28]. The majority of Adiponectin’s biological actions are seen in its HMW form [29]. The High molecular form of adiponectin is the primary active form responsible for insulin-sensitizing actions, whereas the trimeric and hexameric multimers are responsible for the bulk of actions. Total and HMW adiponectin levels have been found to be much greater in females than they are in men. This gender difference in adiponectin concentrations has been seen in both rodents and humans. One possible explanation is that testosterone reduces HMW adiponectin synthesis in men [30]. However, detecting and enriching a specific adiponectin isoform in vivo remains a formidable obstacle [31].
Adiponectin (AdipoQ) Receptors
The protein hormone adiponectin binds to its receptors, which are referred as ADIPOR1 and ADIPOR2, to carry out its biological tasks [19]. Yamauchi was the one who initially discovered both receptors; AdipoR1 and AdipoR2 [32] and Hug has uncovered the identity of another member of cadherin family, which goes by the name T. Cadherin [33]. In skeletal muscle, the adiponectin (AdipoQ) receptor 1 (AdipoR1) has a strong affinity for globular AdipoQ but a low affinity for full-length adiponectin. AdipoR2, on the other hand, is a liver-specific medium receptor for both full-length HMW and globular adiponectin [17]. Adiponectin is along with its receptors (AdipoR1 and AdipoR2) responsible for regulating a variety of bodily functions including inflammatory responses, energy levels throughout the body, insulin sensitivity, and the fatburning mechanism [34]. It has been determined that AdipoR1 is placed on 1p36.13-q41 and 1 E4 chromosomes, whilst AdipoR2 is found on 12p13.31 and 6 F1 chromosomes. Both AdipoR1 and AdipoR2 are G Protein-Coupled Membrane Receptors (GPCR), however their location is unusual compared to that of other receptor proteins. Each receptor has seven transverse membrane patches. Adiponectin interacts to the external C terminus of the adiponectin receptor, whereas the N terminus of the receptor binds to an adaptive protein (also known as APPL1 or “adaptor protein, phosphotyrosine interaction between PH domain and leucine zipper 1”). Skeletal muscle, endothelial cells, synovial fibroblasts, and atrial cells are all known to express AdipoR1 to a significant degree. The expression of AdipoR2 is mostly found in the liver, which has the ability to deactivate PPAR-α receptors, which are known to promote insulin sensitivity. Insulin levels in the blood can affect the expression of adipoRs [35,36].
Insulin Sensitizing Mechanism of Adiponectin
The following are some of the direct and indirect ways in which adiponectin influences insulin sensitivity in type 2 diabetic individuals: First observations of Adiponectin’s impact on insulin sensitivity were made in laboratory mice [37]. Adiponectin plays a significant role in skeletal muscle triglyceride metabolism by lowering adipose tissue triglyceride levels and regulating insulin signalling (Figure 1). Adiponectin also increases production of fatty acid transmission molecules such acyl-coenzyme oxidase and CD36 [38]. Amplification of insulin-stimulated phosphatidylinositol [PI] 3-kinase is associated with a rise in triglyceride levels, the substitution of Glucose-4 Transporter (GLUT-4) and an elevation in glucose uptake, and ultimately insulin resistance.; thus, improved propagation of the insulin signalling pathway is likely to result from reduced triglyceride level in the muscle; Adiponectin stimulates PPAR- receptor phosphorylation activator. Adiponectin boosts energy consumption and fatty acids oxidation by activating PPAR-α. This leads to a drop in the triglyceride level muscle and liver, which eventually leads to an improvement in insulin sensitivity. Adiponectin stimulates AMPK cascade. In conclusion, adiponectin helps to boost the activation and phosphorylation of AMPK in skeletal muscle, which in turn helps to activate beta-oxidation [39]. Therefore, decreased insulin action and Type 2 Diabetes (T2DM) may develop from any genetic change that impairs synthesis of the adiponectin (AdipoQ) protein. Adiponectin treatment in diabetic mice resulted in dramatic enhancement in insulin sensitivity [40,41].
Figure 1: Insulin sensitizing mechanism of adiponectin.
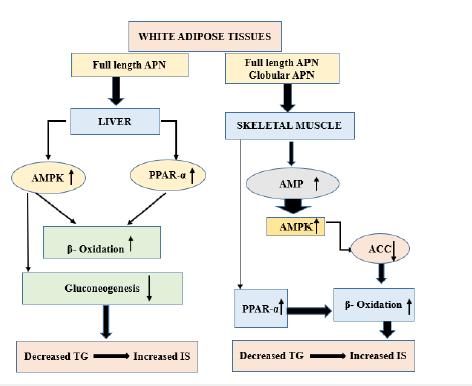
Adiponectinemia, Insulin Resistance and Progression of Type 2 Diabetes
When insulin in the blood has been steadily declining in effectiveness, the pancreas detects insulin resistance, it attempts to compensate by generating more insulin [42]. When insulin does not effectively carry out its metabolic and circulatory responsibilities in its target tissues, this condition is known as insulin resistance [43]. Adiponectin knockout mice (mice without the AdipoQ gene that codes for this protein) have been used to learn more about the function of this adipocytokine from a variety of angles. Reduced plasma acid elimination and FATP 1 concentrations, along with elevated TNF-α levels in plasma and adipose tissue, have been observed in KO mice. Insulin resistance develops in these mice when they are fed a high-glucose, high-fat diet; this is likely due to a reduction in the substrate of the intracellular insulin type 1 receptor, which in turn decreases glucose absorption by some insulin-sensitive tissues (skeletal muscle) [40]. These metabolic aberrations are reversed by restoration of adiponectin expression in this KO mice. Therefore, a lack of adiponectin synthesis may play a role in the pathophysiology of insulin resistance. adiponectin seems to be a hormone sensitizing the activity of insulin. There are a number of clinical trials in humans showing that higher adiponectin levels are protective against insulin resistance and type 2 diabetes in people with normal Body Mass Indexes (BMIs) [44-46]. There is an association between a decreased plasma adiponectin (AdipoQ) level and the development of type 2 diabetes. Animal models of obesity and lipoatrophic mice show that adiponectin increases tissue sensitivity to insulin by decreasing free fatty acid concentrations in the plasma. Adiponectin (AdipoQ) may work via increasing AMPK’s metabolic activity [38]. Its production is downregulated because insulin resistance stresses the endoplasmic reticulum, in which the adiponectin multimer is produced, which in turn stimulates the unfolded protein retorts [47]. Adiponectin secretion and maturation are also suppressed by the oxidative stress and inflammation brought on by obesity [48]. Increased levels of adiponectin are seen in those who have the preventive Pro12Ala mutation of the PPARγ gene. Adiponectinemia is dramatically reduced in individuals with diabetes, insulin resistance, and hypertension who have PPARγ variants with a deleterious dominant impact. It implies that hypoadiponectinemia in individuals raise the risk of developing type 2 diabetes [49]. As a result, we now know that low adiponectinemia is a part of what sets in progression of insulin resistance and type 2 diabetes.
Adiponectin Gene (AdipoQ) Polymorphism in Type 2 Diabetes
The few polymorphisms in the regulatory regions, in intron 2 and exon 2 of the human Adiponectin (AdipoQ) gene may influence AdipoQ gene transcription and secretion. Exon 2 is the translation initiation site. The 5’ untranslated region (5’UTR), Up-strand sequence and intron 1. There are two elements, (SREBP; -431 to -423) or sterol regulatory binding protein and (C/EBP; -230 to -224) or CCAAT/enhancer binding protein, in the promoter region from -676 to +41 that have been shown to be adequate for baseline transcriptional activity [50,51]. Single nucleotide polymorphisms, or SNPs, of the AdipoQ gene have been associated with type 2 diabetes in recent decades. However, there is still debate regarding these studies because of the substantial racial and geographical variances in their findings [52]. Allelic SNPs (single nucleotide polymorphisms) are found in precise numbers throughout the AdipoQ gene sequence [53]. 40 gene loci were found to be associated with the development of type 2 diabetes in a comprehensive investigation of the human genome [54]. Adiponectin gene SNPs rs266729, rs822393, rs3774261, and rs1501299 deliberated mainly with type 2 diabetes in a Kinh Vietnamese population. AdipoQ rs266729 is a potential SNP for type 2 diabetes susceptibility [53]. Decreased High molecular adiponectin, against a baseline of obesity and AdipoQ genetic variations, changed the metabolic profile, increasing the risk for type 2 diabetes [54].
Link between the AdipoQ gene polymorphisms rs1501299 and rs2241766 and type 2 diabetes risk. No association was discovered between the rs1501299 polymorphism of the AdipoQ gene and type 2 diabetes mellitus; nevertheless, the T allele of the polymorphism was recognised as a susceptibility locus for type 2 diabetes mellitus in West Asian ethnicity and exhibited protective outcomes in South Asian individuals [55]. Subjects with impaired glucose tolerance and the G-allele of SNP >45 are more likely to develop type 2 diabetes [56]. Subjects with poor glucose tolerance who also have the T-allele of SNP rs1501299 are at an increased risk of developing type 2 diabetes [57] Variation at position rs17846866 (SNP+10211) in AdipoQ gene has been linked to abnormalities in blood levels of the protein. It is possible that the increased levels of circulating adiponectin in type 2 diabetes are mostly attributable to the TT genotype. However, the G allele may raise the incidence of type 2 diabetes among North Indians. Polymorphism rs17846866 (+10211 T/G) in the AdipoQ gene’s first intron is linked to type 2 diabetes, hypoadiponectinemia and obesity in Asian Indians [58]. Subjects with impaired glucose tolerance and the T-allele of single nucleotide polymorphism rs1501299 had an increased risk of acquiring type 2 diabetes [59] It has been shown that among the people of Southern India, both adiponectin gene variations and haplotypes have a role in the onset of metabolic syndrome, excess body fat, and low levels of the hormone adiponectin. T2DM is strongly associated with the rs3774261 (SNP+712 G/A) gene variant in the South Indian population [60]. The AA genotype of the +712 G/A (rs3774261) SNP was shown to give around 0.65 times decreased risk of developing T2DM. However, only a small number of studies have shown a correlation between this variation and type 2 diabetes and obesity in the Korean population [61].
Adiponectin Gene Expression in Type 2 Diabetes Mellitus
The adiponectin (AdipoQ) possesses features that make it effective against inflammation, atherosclerosis, and diabetes [62]. IL-6, TNF-, and IL-18 are examples of cytokines that are elevated in obese individuals and those who have type 2 diabetes. These cytokines suppress the production of adiponectin. These processes may account for the reduced blood adiponectin expression observed in clinical practice among individuals with obesity and type 2 diabetes [63]. Recent research has demonstrated that adiponectin secretion follows a circadian cycle, with peak production occurring during the day. Some metabolic disorders, including insulin resistance and obesity, have been related to disruptions in the circadian rhythm, but our current understanding of how the circadian clock affects adiponectin expression is limited [64]. Various transcription factors play a crucial role in controlling adiponectin (AdipoQ) gene expression. Moreover, new research indicates that epigenetic processes, including DNA methylation, play a significant role in Adiponectin transcription [65]. Peroxisome Proliferator Activated Receptor (PPAR)-response elements, C/EBP sites, Ebox and FOXO Sterol Regulatory Elements (SREs), are some of the transcription factors that have active binding sites on the adiponectin promoter in both mice and humans. Extremely high levels of PPARγ activity in adipose tissue make it a key positive modulator of adiponectin expression [30]. To inhibit the transcription-activating actions of PPAR-γ, SIRT1 attaches to it by coupling to the nuclear receptor corepressor and silencing the mediator of retinoid and thyroid hormone receptors. Overexpression of SIRT1 was also associated with less fat accumulation and greater lipolysis, which facilitated fat mobilisation in response to dietary restriction [66].
In contrast, inflammatory and obese mediators such Reactive Oxygen Species (ROS), Tumour Necrosis Factor alpha (TNF-a), and Interleukin-6 (IL-6) operate as suppressor of adiponectin expression profiles. In addition, endothelin-1, a crucial vasoconstrictive agent that is elevated in obesity and diabetes, has been shown in in vitro experiments to modulate adiponectin release in 3T3-L1 adipocytes [30]. According to the findings, energy homeostasis is controlled by SIRT1-dependent PPAR-γ deacetylation, which favours energy consumption over energy deposit. Thereby, thiazolidinediones with SIRT1 activator show promise as an obesity induced diabetic treatment [66]. The progression of insulin resistance and the onset of type 2 diabetes has been shown to have an inverse relationship with plasma adiponectin expression [67]. From various studies it was evident that adiponectin is one of the most reliable indicators of type 2 diabetes mellitus [68].
Therapeutic Implications of Adiponectin in Type 2 Diabetes Mellitus
Correlation of adiponectin and type 2 diabetes suggested the various therapeutic roles of AdipoQ in type 2 diabetes. In relation to therapeutic implications, recombinant forms of adiponectin unlike the multimeric adiponectin seen in human adipocytes, the recombinant adiponectin generated by Escherichia coli consists entirely of monomeric AdipoQ [69]. The diverse biological actions of adiponectin are significantly aided by the multimeric complexes [51]. In vivo and in vitro studies have indicated that administration with recombinant adiponectin reduces insulin resistance and protects against the development of metabolic problems and diabetic phenotype [70]. However, posttranslational alterations make it difficult to prepare recombinant adiponectin, despite the fact that it has been proposed as a promising target for producing a therapeutic treatment for treating diabetes. Studies and development in metabolic illnesses have focused on AdipoR agonists for quite some time [71]. Although it would be ideal to design AdipoQ agonists that activate adiponectin receptormediated downstream signaling, doing so is difficult due to difficulties in producing biologically active AdipoQ and optimizing the correct dosage and route of delivery. Okada-Iwabu et al. [72] created AdipoRon, in db/db mice a synthetic adiponectin receptor agonist that may be taken orally. Weight neutrality and improved insulin sensitivity are some of the prometabolic benefits induced by AdipoRon binding to AdipoR1 and AdipoR2 [72].
Research examined if injecting plasmid DNA expressing adiponectin increases its levels. In a non-obese type 2 diabetic mice model, adiponectin infusion raised blood adiponectin levels and lowered glucose [73]. AdipoQ considered a candidate for nonviral delivery of gene. Banerjee et al. [74] produced a gene-based directed nanoparticle formulation that improves insulin sensitivity in T2DM by promoting AdipoQ synthesis in fat tissue and boosting its circulation [74]. Free amino groups on chitosan-oleic acid polymer linked to adipose homing peptide enhanced uptake into adipose tissue. Various investigations have verified this delivery method [75]. Adiponectin gene therapy might treat type 2 diabetes and obesity. Before clinical implication of any gene therapy, specificity, stability, efficiency, safety, and convenience must be carefully considered. New anti-diabetic compounds, thiazolinediones a PPARγ nuclear receptor agonists, have been shown to improve insulin sensitivity, and this improvement is associated with a rise in adiponectinemia [49] in order to understand the physiology of adiponectin most studies are conducted on animal models therefore, further investigations on human subjects are needed from therapeutic perspective.
Conclusion
As a hormone generated from fat cells, adiponectin (AdipoQ) seems to have anti‐atherogenic, insulin‐sensitizing, and antiinflammatory effects that plays a significant role in preventing off insulin resistance, type 2 diabetes, some diverse autoimmune and chronic inflammatory diseases. Subjects with low adiponectin levels are more likely to develop Insulin resistance leading to progression of type 2 diabetes. Adiponectin (AdipoQ) gene possess a susceptibility locus for type 2 diabetes. Genetic variations like DNA polymorphism and expression of Adiponectin (AdipoQ) in serum/plasma can be used as the potential biomarkers for early clinic prediction and diagnosis of type 2 diabetes mellitus.
References
- Schleicher E, Gerdes C, Petersmann A, Müller-Wieland D, Müller UA, et al. (2022) Definition, classification and diagnosis of diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes 127(S 01): S1-S7.
- Akhtar S, Nasir JA, Abbas T, Sarwar A (2019) Diabetes in Pakistan: A systematic review and meta-analysis. Pakistan Journal of Medical Sciences 35(4): 1173-1178.
- Ambachew S, Eshetie S, Geremew D, Endalamaw A, Melku M (2019) Prevalence of type 2 diabetes mellitus among hepatitis C virus-infected patients: A protocol for systematic review and meta-analysis. Systematic Reviews 8(1): 1-4.
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, et al. (2022) IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice 183: 109-119.
- Oh YS, Bae GD, Baek DJ, Park EY, Jun HS (2018) Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Frontiers in Endocrinology 9: 384.
- Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, et al. (2020) Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Research and Clinical Practice 162: 108086.
- DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, et al. (2015) Type 2 diabetes mellitus. Nature Reviews Disease Primers 1(1): 1-22.
- Zhang Y, Pan XF, Chen J, Xia L, Cao A, et al. (2020) Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Diabetologia 63(1): 21-33.
- Nikitin AG, Potapov VY, Brovkina OI, Koksharova EO, Khodyrev DS, et al. (2017) Association of polymorphic markers of genes FTO, KCNJ11, CDKAL1, SLC30A8, and CDKN2B with type 2 diabetes mellitus in the Russian population. Peer J 5: e3414.
- Goyal R, Jialal I, Castano M (2021) Diabetes Mellitus Type 2. StatPearls Publishing.
- Nolan CJ, Prentki M (2019) Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diabetes and Vascular Disease Research 16(2): 118-127.
- Page MM, Johnson JD (2018) Mild suppression of hyperinsulinemia to treat obesity and insulin resistance. Trends in Endocrinology & Metabolism 29(6): 389-399.
- Andrikopoulos S (2010) Obesity and type 2 diabetes: slow down!—Can metabolic deceleration protect the islet beta cell from excess nutrient-induced damage? Molecular and Cellular Endocrinology 316(2): 140-146.
- Cui M, Gao Y, Zhao Y, Pang H, Chen L, et al. (2020) Association between adiponectin gene polymorphism and environmental risk factors of type 2 diabetes mellitus among the Chinese population in Hohhot. BioMed Research International 2020: 6383906.
- Ahlqvist E, Ahluwalia TS, Groop L (2011) Genetics of type 2 diabetes. Clinical Chemistry 57(2): 241-254.
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. Journal of Biological chemistry 270(45): 26746-26749.
- Khoramipour K, Chamari K, Hekmatikar AA, Ziyaiyan A, Taherkhani S, et al. (2021) Adiponectin: structure, physiological functions, role in diseases, and effects of nutrition. Nutrients 13(4): 1180.
- Nguyen TMD (2020) Adiponectin: role in physiology and pathophysiology. International journal of preventive medicine 11(136).
- Jyoti R, Mittal I, Pramanik A, Singh N, Dube N, et al. (2017) T2DiACoD: a gene atlas of type 2 diabetes mellitus associated complex disorders. Scientific Reports 7(1): 6892.
- Smetnev S, Klimushina M, Kutsenko V, Kiseleva A, Gumanova N, et al. (2019) Associations of SNPs of the ADIPOQ gene with serum adiponectin levels, unstable angina, and coronary artery disease. Biomolecules 9(10): 537.
- Yao M, Wu Y, Fang Q, Sun L, Li T, et al. (2016) Association of ADIPOQ variants with type 2 diabetes mellitus susceptibility in ethnic Han Chinese from northeast China. Journal of Diabetes Investigation 7(6): 853-859.
- Talaibekova E, Vinnikov D, Saadanov I, Aldasheva N, Isakova J (2019) ADIPOQ, KCNJ11 and TCF7L2 polymorphisms in type 2 diabetes in Kyrgyz population: A case‐control study. J Cell Mol Med 23(2): 1628-1631.
- Kim S, Lee Y, Kim JW, Son YJ, Ma MJ, et al. (2018) Discovery of a novel potent peptide agonist to adiponectin receptor 1. PLoS One 13(6): e0199256.
- Shapiro L, Scherer PE (1998) The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Current Biology 8(6): 335-340.
- Liu M, Liu F (2014) Regulation of adiponectin multimerization, signaling and function. Best Practice & Research Clinical Endocrinology & Metabolism 28(1): 25-31.
- Sayeed M, Gautam S, Verma DP, Afshan T, Kumari T, et al. (2018) A collagen domain–derived short adiponectin peptide activates APPL1 and AMPK signaling pathways and improves glucose and fatty acid metabolisms. Journal of Biological Chemistry 293(35): 13509-13523.
- Achari AE, Jain SK (2017) Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. International Journal of Molecular Sciences 18(6): 1321.
- Choi HM, Doss HM, Kim KS (2020) Multifaceted physiological roles of adiponectin in inflammation and diseases. International Journal of Molecular Sciences 21(4): 1219.
- Yang J, He L, Gao M, Xiao F, Zhang F, et al. (2021) Collagen β (1-O) galactosyltransferase 2 deficiency contributes to lipodystrophy and aggravates NAFLD related to HMW adiponectin in mice. Metabolism 120: 154777.
- da Silva Rosa SC, Liu M, Sweeney G (2021) Adiponectin synthesis, secretion and extravasation from circulation to interstitial space. Physiology 36(3): 134-149.
- Van Andel M, Heijboer AC, Drent ML (2018) Adiponectin and its isoforms in pathophysiology. Advances in Clinical Chemistry 85: 115-147.
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423(6941): 762-769.
- Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, et al. (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proceedings of the National Academy of Sciences 101(28): 10308-10313.
- Peng YJ, Shen TL, Chen YS, Mersmann HJ, Liu BH, et al. (2018) Adiponectin and adiponectin receptor 1 overexpression enhance inflammatory bowel disease. Journal of Biomedical Science 25(1): 1-13.
- Wang Y, Ma XL, Lau WB (2017) Cardiovascular adiponectin resistance: The critical role of adiponectin receptor modification. Trends in Endocrinology & Metabolism 28(7): 519-530.
- Yang Q, Fu C, Xiao J, Ye Z (2018) Uric acid up regulates the adiponectin adiponectin receptor 1 pathway in renal proximal tubule epithelial cells. Molecular Medicine Reports 17(3): 3545-3554.
- Yadav A, Kataria MA, Saini V, Yadav A (2013) Role of leptin and adiponectin in insulin resistance. Clinica Chimica Acta 417: 80-84.
- Yamauchi T, Kamon J, Minokoshi YA, Ito Y, Waki H, et al. (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine 8(11): 1288-1295.
- Katira A, Tan PH (2016) Evolving role of adiponectin in cancer-controversies and update. Cancer Biology & Medicine 13(1): 101-119.
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, et al. (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine 7(8): 941-946.
- Garcia AL, Steiniger J, Reich SC, Weickert MO, Harsch I, et al. (2006) Arabinoxylan fibre consumption improved glucose metabolism but did not affect serum adipokines in subjects with impaired glucose tolerance. Hormone and Metabolic Research 38(11): 761-766.
- Biswas D, Vettriselvi V, Choudhury J, Jothimalar R (2011) Adiponectin gene polymorphism and its association with type 2 diabetes mellitus. Indian Journal of Clinical Biochemistry 26(2): 172-177.
- Cheng KK, Lam KS, Wang B, Xu A (2014) Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Practice & Research Clinical Endocrinology & Metabolism 28(1): 3-13.
- Hara K, Boutin P, Mori Y, Tobe K, Dina C, et al. (2002) Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes 51(2): 536-540.
- Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, et al. (2003) Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care 26(12): 3226-3229.
- Yamamoto Y, Hirose H, Saito I, Nishikai K, Saruta T (2004) Adiponectin, an adipocyte-derived protein, predicts future insulin resistance: two-year follow-up study in Japanese population. The Journal of Clinical Endocrinology & Metabolism 89(1): 87-90.
- Zhou L, Liu M, Zhang J, Chen H, Dong LQ, et al. (2010) DsbA-L alleviates endoplasmic reticulum stress–induced adiponectin downregulation. Diabetes 59(11): 2809-2816.
- Soares AF, Guichardant M, Cozzone D, Bernoud-Hubac N, Bouzaidi-Tiali N, et al. (2005) Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radical Biology and Medicine 38(7): 882-889.
- Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, et al. (2002) Adiponectin and development of type 2 diabetes in the Pima Indian population. The Lancet 360(9326): 57-58.
- Wang Y, Zhang D, Liu Y, Yang Y, Zhao T, et al. (2009) Association study of the single nucleotide polymorphisms in adiponectin-associated genes with type 2 diabetes in Han Chinese. Journal of Genetics and Genomics 36(7): 417-423.
- Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, et al. (2003) Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin: implications for metabolic regulation and bioactivity. Journal of Biological Chemistry 278(11): 9073-9085.
- Suriyaprom K, Phonrat B, Namjuntra P, Harnroongroj T, Tungtrongchitr R (2010) The-11377C>G adiponectin gene polymorphism alters the adiponectin concentration and the susceptibility to type 2 diabetes in thais. International Journal for Vitamin and Nutrition Research 80(3): 221-224.
- Truong S, Tran NQ, Ma PT, Hoang CK, Le BH, et al. (2022) Association of ADIPOQ single-nucleotide polymorphisms with the two clinical phenotypes type 2 diabetes mellitus and metabolic syndrome in a Kinh Vietnamese population. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 15: 307-319.
- Palit SP, Patel R, Jadeja SD, Rathwa N, Mahajan A, et al. (2020) A genetic analysis identifies a haplotype at adiponectin locus: association with obesity and type 2 diabetes. Scientific Reports 10(1): 2904.
- Dong Y, Huang G, Wang X, Chu Z, Miao J, et al. (2020) Meta-analysis of the association between adiponectin SNP 45, SNP 276, and type 2 diabetes mellitus. PLoS One 15(10): e0241078.
- Zacharova J, Chiasson JL, Laakso M, STOP-NIDDM Study Group (2005) The common polymorphisms (single nucleotide polymorphism [SNP]+ 45 and SNP+ 276) of the adiponectin gene predict the conversion from impaired glucose tolerance to type 2 diabetes: the STOP-NIDDM trial. Diabetes 54(3): 893-899.
- Ando D, Hosaka Y, Suzuki K, Yamagata Z (2011) The influence of adiponectin G276T gene polymorphism on changes in total and high molecular weight adiponectin levels by exercise training. Health 3(12): 737-741.
- Vimaleswaran KS, Radha V, Ramya K, Babu HNS, Savitha N, et al. (2008) A novel association of a polymorphism in the first intron of adiponectin gene with type 2 diabetes, obesity and hypoadiponectinemia in Asian Indians. Human Genetics 123(6): 599-605.
- Rasmussen-Torvik LJ, Pankow JS, Jacobs DR, Steinberger J, Moran A, et al. (2009) The association of SNPs in ADIPOQ, ADIPOR1, and ADIPOR2 with insulin sensitivity in a cohort of adolescents and their parents. Human Genetics 125(1): 21-28.
- Ramya KA, Ayyappa S, Ghosh V, Radha MV (2013) Genetic association of ADIPOQ gene variants with type 2 diabetes, obesity and serum adiponectin levels in south Indian population. Gene 532(2): 253-262.
- Cheong MY, Bang OS, Cha MH, Park YK, Kim SH, et al. (2011) Association of the adiponectin gene variations with risk of ischemic stroke in a Korean population. Yonsei Medical Journal 52(1): 20-25.
- Seo Y, Shin TH, Kim HS (2019) Current strategies to enhance adipose stem cell function: an update. International Journal of Molecular Sciences 20(15): 3827.
- Atzmon G, Pollin TI, Crandall J, Tanner K, Schechter CB, et al. (2008) Adiponectin levels and genotype: a potential regulator of life span in humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 63(5): 447-453.
- Krause MP, Milne KJ, Hawke TJ (2019) Adiponectin—Consideration for its role in skeletal muscle health. International Journal of Molecular Sciences 20(7): 1528.
- Ott R, Stupin JH, Melchior K, Schellong K, Ziska T, et al. (2018) Alterations of adiponectin gene expression and DNA methylation in adipose tissues and blood cells are associated with gestational diabetes and neonatal outcome. Clinical Epigenetics 10(1): 1-12.
- Kitada M, Koya D (2013) SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes & Metabolism Journal 37(5): 315-325.
- Prates RE, Beretta MV, Nascimento FV, Bernaud FR, de Almeira JC, et al. (2016) Saturated fatty acid intake decreases serum adiponectin levels in subjects with type 1 diabetes. Diabetes Research and Clinical Practice 116: 205-211.
- de Luis DA, Izaola O, Primo D, Gómez-Hoyos E, Ortola A, et al. (2019) Role of rs1501299 variant in the adiponectin gene on total adiponectin levels, insulin resistance and weight loss after a Mediterranean hypocaloric diet. Diabetes Research and Clinical Practice 148: 262-267.
- Fang H, Judd RL (2018) Adiponectin regulation and function. Comprehensive Physiology 8(3): 1031-1063.
- Shi W, Guo Z, Ji Y, Feng J (2020) The protective effect of recombinant globular adiponectin on testis by modulating autophagy, endoplasmic reticulum stress and oxidative stress in streptozotocin-induced diabetic mice. European Journal of Pharmacology 879: 173132.
- Otvos L (2019) Potential adiponectin receptor response modifier therapeutics. Frontiers in Endocrinology 10: 539.
- Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, et al. (2013) A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503(7477): 493-499.
- Nan MH, Park JS, Myung CS (2010) Construction of adiponectin-encoding plasmid DNA and gene therapy of non-obese type 2 diabetes mellitus. Journal of Drug Targeting 18(1): 67-77.
- Banerjee A, Sharma D, Trivedi R, Singh J (2020) Treatment of insulin resistance in obesity-associated type 2 diabetes mellitus through adiponectin gene therapy. International Journal of Pharmaceutics 583: 119357.
- Thovhogi N, Sibuyi N, Onani MO, Meyer M, Madiehe AM (2018) Peptide-functionalized quantum dots for potential applications in the imaging and treatment of obesity. International Journal of Nanomedicine 13: 2551-2559.
© 2022 Saima Sharif. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






