- Submissions

Full Text
Intervention in Obesity & Diabetes
A Novel Method to Quantify Carbohydrate Routine Consumption: A Reliable, Valid, Sensitive and Simple Alternative to 24-Hour Dietary Recall
Daniel J Cox1*, Matthew Moncrief1, Harsimran Singh2, Anne Diamond1 and Anthony L McCall3
1University of Virginia School of Medicine, Center for Behavioral Medicine Research, USA
2Mary & Dick Allen Diabetes Center, Hoag Memorial Hospital, USA
3University of Virginia School of Medicine, Endocrinology and Metabolism, USA
*Corresponding author:Daniel J Cox, University of Virginia Health System, Center for Behavioral Medicine Research, PO Box 800223, Charlottesville, VA 22908, USA
Submission:October 04, 2021;Published: November 09, 2021

ISSN 2578-0263Volume5 Issue4
Abstract
Objective: Growing evidence suggests postprandial hyperglycemia, driven largely by carbohydrate consumption, adversely affects A1c and cardiovascular health. The standard for quantifying carbohydrate intake is 24-hour dietary recall (ASA24). While sophisticated in terms of quantifying grams of micro and macro nutrients, it is often not practical in most clinical and some research settings. A simpler alternative is needed.
Research design and methods: We developed the Carbohydrate Routine Consumption (CRC) scale, which samples weekly servings of 16 common high and low glycemic load foods and takes 5 minutes to complete and score. We administered the CRC and the ASA24 to 204 adults with type 2 diabetes.
Results: The CRC was reliable, correlated with the ASA24 and had similar construct and discriminant validity.
Conclusion: The CRC is psychometrically sound and easily administered and scored by clinicians and researchers to document routine carbohydrate consumption and change in consumption.
Introduction
Given that carbohydrates are a major contributor to Post Prandial Glucose (PPG) [1], which in turn is a major contributor to A1c [2], and possibly an independent contributor to diabetic cardiovascular complications [3], researchers and clinicians need to quantify carbohydrate consumption. The 24-hour dietary recall (ASA24) [4] is considered the gold standard to quantify carbohydrate intake, but it is not practical for clinicians and many researchers. For standardization, the ASA24 requires a trained examiner conducting three days of 30-minute scheduled telephone interviews using an online form to enter all the nutrients consumed in the past 24 hours, their volume and how the foods were prepared. The data is analyzed by the ASA24 server, which calculates all macro and micronutrients, and the researcher must make a batch request for all recalls completed in a study, the results of which are typically available a day later. Consequently, we developed a simpler, self-report questionnaire that only quantifies servings of Carbohydrates Routinely Consumed (CRC) in the previous week (Figure 1).
Figure 1:Carbohydrates routinely consumed scale.

Introduction
Instrument
To solicit a representative sampling of routinely eaten carbohydrates, the CRC asks: “How many servings of the following foods do you eat in an average week? A serving size is about the size of a deck of cards. A large or a ‘supersized’ serving is equal to two servings”. For brevity and ease of use, the CRC does not list all food varieties and their preparations. Instead, it presents 16 classes of foods with a glycemic load>10 (items 1-16, CRCHGL), e.g. “potatoes like mashed, baked, fried, white, red, sweet, potato soup, potato pancakes, etc.”, and 15 classes of foods with a glycemic load<7 (items 17-31, CRCLGL), e.g. ”green vegetables like peas, spinach, kale, broccoli, etc.” [1]. The CRCHGL and CRCLGL scores are the sum of the reported servings consumed in an average week for items 1-16 and 17-32, respectively. We performed pilot testing to clarify items and affirm initial reliability. This report focuses on the CRCHGL. We hypothesized that the CRCHGL would have: 1) significant test-retest reliability, 2) concurrent validity (a moderate correlation with the ASA24 grams of carbohydrate), 3) construct validity (a positive correlation with A1c, BMI, calorie intake, depressive symptoms [eating comfort foods]), and 4) discriminant validity (a decreased response to PPG-lowering interventions, but not to a weight loss intervention, and a pre-post change in A1c correlation only in the PPG-lowering intervention). As a control, we hypothesized that CRCLGL would not demonstrate such relationships except with respect to calories. Since calories come from both low and high glycemic load foods, we hypothesized CRCLGL would mildly correlate with the ASA24 total calories consumed.
Participants
The sample consisted of 204 adults with type 2 diabetes from a randomized clinical trial [5] with a mean±SEM age=56.0±11.7, duration of disease=5.2±3.0, BMI=34.7±6.4, A1c=8.1±1.3, gender=58.8% female.
Procedure
At baseline, participants completed the CRC and the Patient Health Questionnaire (PHQ9) [6] to measure symptoms of depression. A1c and BMI were also measured. The following week, participants were called on two weekdays and one weekend day to administer the ASA24. A subgroup of 20 participants repeated the CRC seven days after their initial completion, to assess test-retest reliability. Participants were then randomized into a 2-month lifestyle intervention for diabetes that compared 6 hours of weight reduction (caloric restriction, N=36) to 6 hours of Glycemic Excursion Minimization (GEM, N=168). GEM focuses on reducing rise in post-nutrient BG with low glycemic load foods and hastening it’s recovery by increasing routine physical activity. GEM participants were divided into three sub-groups that varied the amount of blood glucose feedback they received concerning the impact of food and activity choices. We repeated the baseline assessment three months after treatment concluded.
Results
Participants reported eating 32.9±16.2 servings/week of CRCHGL foods, similar to that reported previously (33.3±15.9) [7]. Over three days of ASA24 reporting, an average of 223±58.6g of carbohydrates were eaten (54% of participants’ daily nutrient intake). The standard errors for these measures were 1.13 and 7.18 respectively. The control variable, CRCLGL, demonstrated good test retest reliability (r=0.82, p<0.001) and correlated with total calories consumed (r=0.20, p=0.03), but did not relate significantly to the CRCHGL, the ASA24, or to any of the validity variables as hypothesized.
Reliability
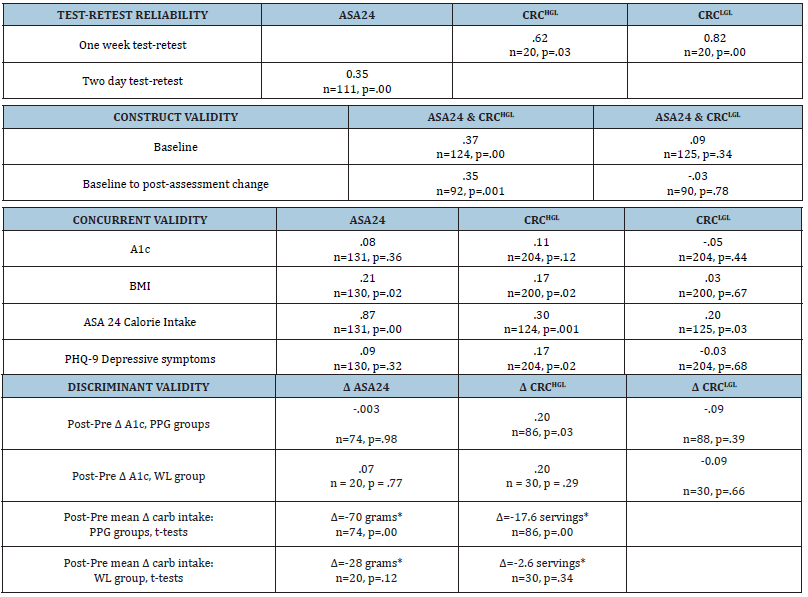
The CRCHGL (r=0.62) demonstrated significant test-retest reliability, greater than the ASA24 from day 1 to 3 (r =0.35. Table 1).
Table 1:Correlation coefficients and t-tests of change scores (*) demonstrating the reliability and validity of the CRC.
Construct validity
Baseline CRCHGL correlated significantly with the ASA24’s total carbohydrates eaten (r=0.37, p<0.001). The change in carbohydrates from baseline to post-treatment follow-up was correlated for these two metrics (r= 0.35, p<0.001).
Concurrent validity
The CRCHGL correlated significantly with BMI, calories consumed, and depressive symptoms. The ASA24’s total carbohydrates correlated significantly with BMI and total calories (Table 1).
Discriminant validity
Only the change in CRCHGL correlated significantly with change in A1c reduction for PPG groups but not for the WL group. Only the PPG groups reduced carbohydrate consumption, (t-tests) as quantified by both the CRCHGL and the ASA24 (Table 1)
Conclusion
Compared to the time-consuming administration and short sampling period (nutrients consumed in the past 24 hours) of the ASA24, the CRCHGL is a much simpler and more focused metric for routine carbohydrate consumption. The psychometric properties of the CRCHGL are similar to those of the ASA24. In addition, the CRCHGL relates to depressive symptoms. Reductions in the CRCHGL are related to reductions in A1c, while reductions in the ASA24 are not. While construct validity correlates are anticipated to be moderate, they were low in the present data set. The only metric favoring the ASA24 is its higher relationship to total calories consumed. This higher correlation may in part, be attributed to the ASA24’s total carbohydrates and total calories coming from the same interview, reviewing the same three days, while the CRCHGL reflects eating behavior from the week before [8]. It is also important to note that the current sample was adults with type 2 diabetes, so these findings can only be extrapolated to this patient group. Further validation of the CRCHGL comes from a recent study where GEM was similarly administered to a group of adults newly diagnosed with T2d. In that study CRC HGL was similarly reduced by GEM (p<0.001) and pre-post change in CRC correlated with reduction in A1c (r=0.66). These data suggest that clinicians and researchers working with adults having type 2 diabetes, can use the CRCHGL to quickly and easily gain insight into a person’s routine consumption of high glycemic load foods and whether or not it changes over time. Based on the CRC results from the current and a previous study, a patient consuming 49 servings/week (one SD above the mean) would be ingesting a high carbohydrate load. A patient who decreased their consumption by 16 servings would be substantially reducing their routine carbohydrate ingestion. Further, the CRC can be used as an education tool for a low carbohydrate diet, i.e. avoid foods 1-16 and embrace foods 17- 31. However, if a clinician/investigator wants a broad nutritional analysis of micro and macro nutrients on specific days, the ASA24 is required.
Acknowledgment
Funding: A grant from NIH/NIDDK funded this effort and a grant from DexCom, Inc. provided Platinum IV CGM sets, supplies, and partial financial support to conduct this project.
Conflict of Interest
D.J.C. received a grant from NIH/NIDDK and from DexCom, Inc., which provided Platinum IV CGM sets, supplies, and partial financial support to A.D. for this project. The sponsor was not involved in the design or conduct of the study, or in the preparation of this manuscript. D.J.C. has served as a consultant to Pfizer, Merck, and Sanofi. The University of Virginia received support for contract research from Eli Lilly and Sanofi conducted by A.L.M. No other potential conflicts exist for this article.
Author Contributions
D.J.C. wrote the manuscript, researched data, and is the guarantor of this work. M.M. analyzed and researched data. A.D. ran subjects and reviewed/edited the manuscript. A.M. contributed to the discussion and reviewed/edited the manuscript.
References
- Augustin L, Kendall C, Jenkins D, Willett W, Astrup A, et al. (2015) Glycemic index, glycemic load and glycemic response: an International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis 25(9): 795-815.
- Liu A, Most M, Brashear M, Johnson W, Cefalu W, et al. (2012) Reducing the glycemic index or carbohydrate content of mixed meals reduces postprandial glycemia and insulinemia over the entire day but does not affect satiety. Diabetes Care 35(8): 1633-1637.
- Ceriello A, Davidson J, Hanefeld M, Leiter L, Monnier L, et al. (2006) Postprandial hyperglycaemia and cardiovascular complications of diabetes: An update. Nutr Metab Cardiovasc Dis 16(7): 453-456.
- Subar A, Kirkpatrick S, Mittl B, Zimmerman T, Thompson F, et al. (2012) The automated self-administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet 112(8): 1134-1137.
- Cox DJ, Banton T, Moncrief M, Conaway M, Conaway M, et al. (2020) Glycemic excursion minimization in the management of type 2 diabetes: A novel intervention tested in a randomized clinical trial. BMJ Open Diabetes Res Care 8(2): e001795.
- Kroenke K, Spitzer R, Williams J (2001) The PHQ‐9: Validity of a brief depression severity measure. J Gen Intern Med 16(9): 606-613.
- Cox D, Taylor A, Singh H, Moncrief M, Diamond A, et al. (2016) Glycemic load, exercise, and monitoring blood glucose (GEM): A paradigm shift in the treatment of type 2 diabetes mellitus. Diabetes Res Clin Pract 111: 28-35.
- Oser TK, Cucuzzella M, Cox DJ, Stasinopoulos M, Moncrief M, et al. (2021) The use of continuous glucose monitoring integrated with an innovative lifestyle intervention to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: A pilot study. Annals of Family Medicine.
© 2021 Daniel J Cox. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






