- Submissions

Full Text
Intervention in Obesity & Diabetes
Ten Weeks Protocol of Physical Exercise for an Elderly Person Diagnosed with Diabetes Mellitus and Hypertension: An Evidence-Based Case Report
Campos pereira WV1,2*, de Almeida DL1,2, Aguiar DD1, Reis Medeiros RL1, Madureira Sabino TB1 and Martins Vancea DM1,2,3
1Research group in Physical Exercise and Non-Transmissible Diseases, Brazil
2University of Pernambuco – UPE, Brazil
3Permanent professor at the Physical Education Program at the Federal University of Pernambuco, Brazil
*Corresponding author:William Valadares Campos Pereira, PhD in Biological Sciences, University of Pernambuco, Recife, Brazil. Mail ID: william.valadares@yahoo.com.br
Submission:September 11, 2021;Published: September 20, 2021

ISSN 2578-0263Volume5 Issue4
Abstract
The prescription of physical exercises for groups of people who need special care during prescribing and monitoring is an area of Physical Education and Exercise Physiology that needs more scientific reports, as evidence-based practical interventions can help professionals who work with exercise prescription for their audiences. In this work, we report the prescription of physical exercises for an elderly aged 82, diagnosed with type 2 diabetes mellitus, diabetic neuropathy and nephropathy, arterial hypertension, and a history of stroke. The choice of exercises was based mainly on the exercise recommendations of the Brazilian Society of Diabetes, Brazilian Society of Arterial Hypertension, World Health Organization, and the possibilities of available materials and adequacy of scientific information to the student’s reality at home.
Keywords:Diabetes mellitus; Physical exercise; Hypertension; Diabetic neuropathy; Diabetic nephropathy
Introduction
The World Health Organization (WHO) considers elderly persons aged 65 years or over and for this audience, physical activity confers several health benefits: it reduces mortality levels, as well as the risk of chronic degenerative diseases, such as type 2 diabetes mellitus (DM2), arterial hypertension and cancer; in addition, it brings numerous benefits to mental health, reducing symptoms of anxiety and depression. Other expected benefits are improved cognitive health, sleep, and anthropometric factors [1]. The aging process is often linked to reduced physical capacity levels and cognitive impairment; scientific evidence demonstrates that physical activity improves physical function and generates benefits related to cognition and socialization in the elderly [1,2]. DM2 is a pathology commonly associated with obesity and with the aging process, is characterized by insulin resistance and chronic hyperglycemia, which in turn result in an increase in reactive oxygen species production, advanced non-enzymatic glycolysis products (AGEs) and triggering of inflammatory cascades that causes damage to various organs and tissues, which can lead to the diagnosis of chronic complications related to diabetes, such as peripheral neuropathy, autonomic neuropathy, nephropathy, retinopathy, among others [3]. The current approaches to management of DM2 and some of its consequences such as diabetic neuropathy focus on improving glycemic control, lifestyle modifications management of neuropathic pain. The optimal therapeutic approach for patients with DM2 includes lifestyle interventions, specifically diet and exercise, coupled with optimal lipid and blood pressure control [4].
In addition to other strategies, different drugs are commonly used to control type 2 diabetes, among which the most common are oral hypoglycemic agents such as metformin and thiazolidinediones (rosiglitazone, pioglitazone), which have different mechanisms for improving insulin sensitivity and increasing the endogenous production of this hormone. However, individuals with DM2 may also need regular insulin given that in some cases, in addition to insulin resistance, some of them show low or impaired endogenous insulin production. Individuals with type 1 diabetes (DM1) or DM2 who use insulin or other secretagogues need special care during physical exercise practices, especially concerning the prevention of hyperglycemic conditions during or after the exercise session [5]. One of the most common associated symptoms present in DM2 patients is elevated systemic arterial hypertension, which is considered as maximum and minimum when pressure values are equal to or greater than 140/90mmHg, and these values are sustained over the days when evaluated by laboratory tests. Hypertension is another chronic disease, often associated with the aging process, characterized by high blood pressure levels in the arteries, requiring therapeutic and non-therapeutic treatment for effective control [6]. Some non-pharmacological strategies, such as changing the lifestyle, healthy eating, reduced use of sodium in foods, and regular physical exercise, are essential for the success of the treatment. Hypertensive patients who reach the recommended physical exercise practice suggested for health, reduce the risk of mortality by 27 to 50%, even those who fail to meet the ideal exercise recommendations and perform lower levels, also have beneficial effects [7]. The WHO suggests that older adults should do between 150 and 300 minutes of aerobic physical activity of moderate intensity throughout the week or at least 75-150 minutes of aerobic physical activity of vigorous intensity. The equivalent combination of moderate and vigorous activity during the week can also be an option, all of the above recommendations can generate substantial health benefits for the elderly [1]. In addition to the recommendations about aerobic exercise for this population, the WHO also recommends inclusion of muscle-strengthening activities at moderate levels or with greater intensity, and that involve all major muscle groups, with a minimum weekly frequency of 2 days, as they provide additional health benefits [1]. Such recommendations are supported by the guidelines of the Brazilian Society of Diabetes (SBD) and by the Brazilian Guidelines on Hypertension.
However, the guidelines also suggest specific recommendations that require attention to some determining factors, such as blood glucose levels and blood pressure. A compilation of these recommendations is presented in Table 1. The above recommendations must be considered by the Physical Education or health professional who is working in physical rehabilitation through physical exercise, thus ensuring the safety and evolution of the student/patient. Considering the scientific evidence and recommendations of the related guidelines, this study aimed to report the prescription and follow-up of physical exercise, for eight weeks, in an 83-year-old individual who has a diagnosis of DM2, peripheral neuropathy, nephropathy, and systemic arterial hypertension in the home environment.
Table 1: Compiled recommendations for individuals with diabetes and high blood pressure.

Material and Methods
Sample
Data collection occurred after the volunteer signed the free and clarified declaration, thus authorizing that the methods described here could be performed and disseminated in scientific circles. The sample consisted of 1 male, aged 83 years, diagnosed with DM2, peripheral neuropathy, nephropathy, systemic arterial hypertension, and a history of stroke. Makes regular use of medications to control blood glucose (including NPH insulin), high blood pressure, and kidney function. The patient has difficulty in walking due to a history of stroke, an outcome that compromised the balance and movements of the lower limbs (especially the ankle and feet region). Due to the difficulty in walking and the consequent risk of falling, the patient needs a walker in the home environment and outside. After the musculoskeletal assessment, it was observed: mobility and strength compromised in the right ankle and adjacent muscles, mobility, and strength slightly compromised in the left ankle and adjacent muscles. General muscle strength with physiological levels preserved for the age group in knee extensors, hip flexors, thigh adductors and abductors, and upper limbs.
Methods
During the treatment, blood glucose and systemic blood pressure were measured before and after the exercise sessions. The Accu-Chek Active Roche glucometer was used to evaluate blood glucose, and glycemic values between 100 and 250mg/ dl were considered appropriate for physical exercise. In cases where blood glucose was lower than 100mg/dl pre-exercise, the individual ingested 15g of carbohydrates, and if blood glucose was above 250mg/dl, the exercises were postponed as recommended by the practical manual on physical exercise for people with DM2, a material developed by the SBD [8] and only stretching and hydration of the feet were performed, due to excessive skin dryness in the lower limbs. The Premium brand sphygmomanometer and stethoscope were used to measure systemic blood pressure. Blood pressure values up to 160/105mmHg were considered appropriate for physical exercise, and in the case of higher values, the individual only did stretch and hydration of the feet. In both cases of not executing the exercise session, the feet were hydrated with dersani oil or sunflower oil as recommended in the material provided by the SBD. The recommendation is to avoid applying the oil between the toes or soles of the feet to prevent fungi accumulation and reduce the risk of falling due to slipping [9].
Physical exercise protocol
The physical exercise sessions occurred in the home environment, with the individual seated in a chair, and the walker was used as a support for standing or walking exercises. As recommended by the guidelines presented above, flexibility, balance, agility, strength, and aerobic exercises were prioritized. Flexibility exercises involved stretching the hamstrings, gluteus medius, gastrocnemius, pectorals, and lateral and posterior torso muscles. The passive and active stretching methods were used, with the maintenance of 30 seconds per stretching set, two sets of each. For the strength exercises, an elastic band (mini theraband) with medium tension was used, for the exercises to strengthen the tibialis anterior and gastrocnemius (Figures 1A & 1B), gluteus medius (Figure 2), and knee extensors (Figure 3). For the strengthening of the thigh adductors, a pilates toning ring with medium tension was used (Figure 4). For the work of brachial biceps strength, we used 2 rubber bands with medium resistance and with grippers on the extremities (Figure 5), with this same material, the bench press exercise was performed. To perform the hip flexor balance and strength exercises, hip flexion was performed in a standing position, alternately, with support on a chair or walker (Figure 5). For all strength exercises, 2 to 3 sets of 8 to 12 repetitions with 90-second intervals were performed.
A small pilates ball was used for lower and upper limb agility exercises. The student sitting on the chair was encouraged to dominate the ball with their feet and perform the pass, alternating between right and left (Figures 6A & 6B). The agility of the upper limbs was exercised through throws with the hands, where the student needed to receive the ball and throw in sequence, an average time of 8 minutes. The aerobic work was carried out through locomotion in the building’s hall, with the help of a walker, with an average time of 15 minutes. The sessions lasted an average of 60 minutes total, all exercises and sessions were prescribed and monitored by a Physical Education professional, specialized in Diabetes and exercise prescription for special groups with a Ph.D. level and superior experience for ten years with the diabetic public.
Figure 1: Strengthening of anterior tibialis and gastrocnemius with mini theraband. Source: Figure elaborated by the author. (A) strengthening of the anterior tibialis muscle. (B) strengthening of the gastrocnemius muscle.
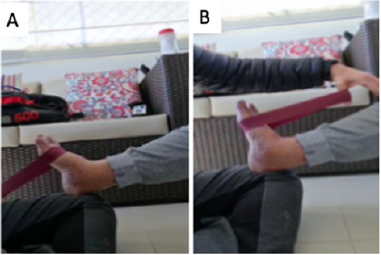
Figure 2: Strengthening of the gluteus medialis with mini theraband. Source: photo was elaborated by the author. The red arrow indicates the position of the theraband.
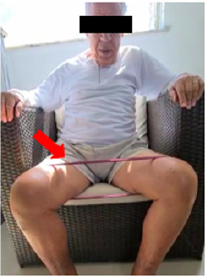
Figure 3: Strengthening of the thigh adductors with toning pilates ring. Source: Photo elaborated by the author.
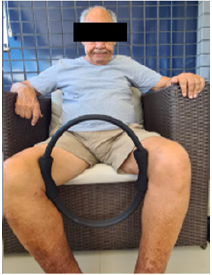
Figure 4: Strengthening of elbow flexors muscles. Source: Photo elaborated by the author.
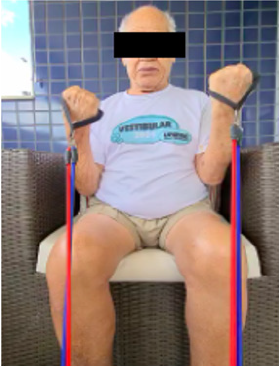
Figure 5: Strengthening of the hip flexors in standing position alternated with walker support. Source: Photo elaborated by the author.
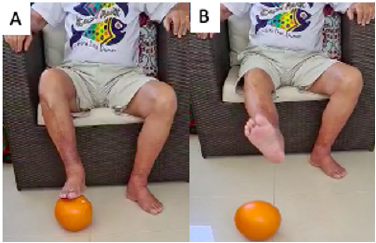
Figure 6: Lower limb agility exercise with pilates ball: alternating mastery and passing. Source: Photo was elaborate by the author. (A) mastery (B) passing.
Results
Over the ten weeks of follow-up, a total of 20 exercise sessions were performed. In three of them, as a safety measure, strength or aerobic exercises were not applied, due to higher blood pressure values than recommended by the Brazilian guidelines for arterial hypertension; in these cases, hydration of the lower limbs (Figures 7A & 7B) and stretching (Figure 8). The patient had forty measurements of capillary blood glucose (20 measurements before and 20 measurements immediately after the sessions) and 40 measurements of blood pressure (20 measurements before and 20 measurements immediately after the sessions) collected. Considering the reference values suggested by the SBD, people with insulin-treated diabetes should exercise preferentially, with blood glucose levels above 100mg/dl and below 250mg/dl [5]. After blood glucose values analyze, the patient was able to perform the training sessions 100% of the time, since the blood glucose obtained was between the reference values (Graph 1). Considering the reference values suggested by the Brazilian guidelines on Arterial Hypertension, the patient was able to perform the training session when the blood pressure, before physical exercise, was below 160/105mmHg. Three exercise sessions were not performed due to signs of uncontrolled blood pressure, values above the recommended (Graphs 2 & 3).
Figure 7: Hydration of lower limbs. Source: Photo elaborated by the author. (A) Prior to hydration; lower limb with broad appearance of dryness. (B) After hydration; lower limb with less dryness appearance.
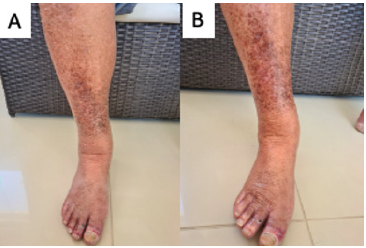
Figure 8: Hydration of lower limbs. Source: Photo elaborated by the author. (A) Prior to hydration; lower limb with broad appearance of dryness. (B) After hydration; lower limb with less dryness appearance.
Graph 1: Blood glucose values prior and immediately after the exercise sessions.
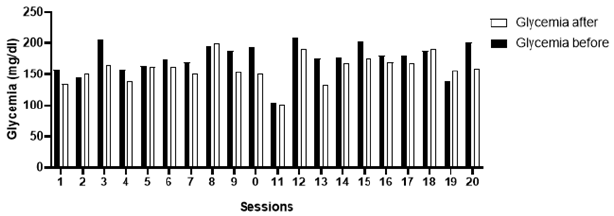
Graph 2: Systolic blood pressure measured prior and immediately after the exercise session.
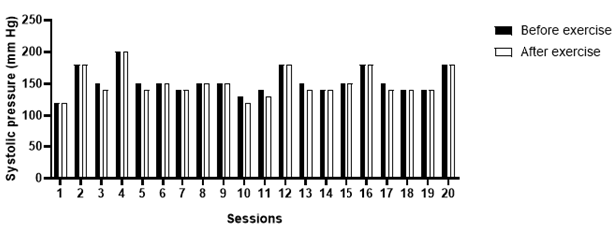
Graph 3: Diastolic blood pressure measured prior and immediately after the exercise session.
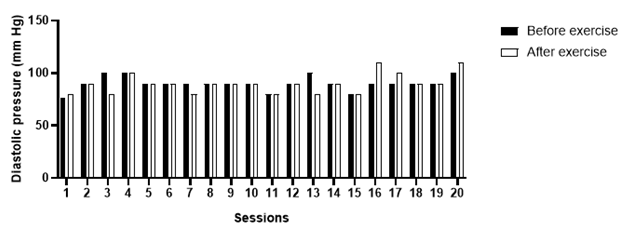
Discussion
The patient had a glycemic profile compatible with the practice of physical exercise in all sessions. During follow-up, he had baseline values above 100mg/dl and below 250mg/dl. In a single session (number 11), the teacher suggested ingesting 15g of carbohydrates after the end, to minimize the chance of hypoglycemia, considering that the student’s next meal was not close. It is important to highlight that the patient followed a regular diet recommended by his endocrinologist. Diet control is one of the main challenges in DM2 treatment and it must be observed in all patients with a high level of stringency. According to [5], the ideal components of a diet for the treatment of DM2 patients is: 45-60% carbohydrates (with possible individual adjustments depending on the patient demands); 20-35% total fat (preferably with monounsaturated and polyunsaturated fats); 15-20% proteins; 20g/1,000Kcal of dietary fibers. In the present case, the amount of carbohydrates was adjusted in association with the physical exercise protocols to better meet the patient energy expenditure needs. Works from [10] demonstrated that appetite regulation and insulin sensitivity modulation are fundamental for promoting anti-aging effects on cells, mainly through activation of anti-aging genes, such as Sirt1 and Forkhead box proteins (FOXO1/FOXO3a). The importance of anti-aging genes in insulin sensitivity and diabetes maintenance has been reviewed by the works of [11] where it is highlighted the importance of appetite regulation and curbing diet programs to further improve the treatment to diabetic patients that suffer from comorbidities associated with obesity and insulin resistance, such as Non-alcoholic Fatty Liver Disease (NAFLD).
The glycemic reductions observed after most exercise sessions can be explained due to the increase in insulin sensitivity caused by the physical exercise, a mechanism that might involve the increased expression of the glucose transporter gene, GLUT4 [12]. Immediate reductions in blood glucose are more frequent in light and moderate exercise [13] and when performing strength exercise before aerobic exercise [14]. High-intensity exercise increases the expression of counterregulatory hormones, mainly catecholamines and cortisol, which momentarily increase blood glucose due to increased intensity-induced glycogenolysis [15,16]. The results demonstrated that the patient presents constant fluctuations in blood pressure values, and a return to the specialist physician (cardiologist) was recommended to assess a possible need for medication adjustment. As a result of blood pressure variations, in four sessions it was decided not to perform the strength and flexibility exercises. In those situations, only passive mobilization of the lower limbs, massage, and hydration of the legs was performed. In most sessions, no explicit manifestations of post-exercise hypotension were observed. This finding follows the literature that investigated physical exercise as an alternative therapy for the treatment of resistant hypertension, and in some of the papers, promising results are reported [17] and [18]. The mechanisms involved in post-exercise hypotension are still under investigation, and some studies showed a correlation between the exercise intensity and its hypotensive effect. As suggested by [17], vigorous-intensity exercises could generate a greater hypotensive response after the sessions in comparison with moderate or low intensity exercises, possibly due to a long-lasting counterregulatory mechanism. However, the authors also saw a hypotensive response in light sessions, which demonstrates that there is still much to be researched regarding the hypothesis that post-exercise hypotension presents a dose-response relationship with intensity [18]. After the follow-up of ten weeks of physical exercise and musculoskeletal reassessment (based on the expertise of the Physical Education professional, a diabetes specialist), a few remarks followed:
a. No improvement in mobility and strength in the left ankle and adjacent muscles. Significant gains in mobility and strength in the right ankle and adjacent muscles, lower limb less affected after the stroke outcome. Significant improvements in strength in the upper and lower limbs, with emphasis on gains in knee extensors, thigh adductors, thigh abductors, hip flexors, and tibialis anterior.
b. Significant gains in motor skill and reflexes, with the performance of exercises with the ball, both in the lower limbs and in the upper limbs.
c. No balance gains were observed in the standing position, perhaps due to the sequelae already established by the stroke event.
d. Improvements in walking with the walker were observed, in addition to increased skill and coordination. However, we cannot infer any fitness improvement. After analyzing all the outcomes presented over the ten weeks of physical exercise, it is noteworthy that the sessions were carried out within the parameters of glycemic and blood pressure safety, thanks to the recommendations found in the specialized guidelines.
Conclusion
We conclude that the specialized guidelines mentioned here, present safe exercise recommendations for the public with diabetes and systemic arterial hypertension. Such guidelines offer didactic tools, which must be followed by Physical Education professionals and other prescribers who use physical exercises as rehabilitation. It is noteworthy the importance of measuring blood glucose and blood pressure at each session, these being used as a guide for the performance or not of sessions, ensuring the health of practitioners. In addition, such records can and should be considered by the physicians involved in the student/patient’s treatment, evaluating possible modulations that may draw attention to non-physiological changes that may be subject to intervention.
References
- World Health Organisation (2020) WHO guidelines on physical activity and sedentary behaviour, Web Annex, Evidence Profiles. Switzerland.
- Neviani F, Murri MB, Mussi C, Triolo F, Toni G, et al. (2017) Physical exercise for late life depression: Effects on cognition and disability. Int Psychogeriatr 29(7): 1105-1112.
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, et al. (2018) IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138: 271-281.
- Feldman EL, Callaghan BC, Pop BR, Zochodne DW, Wright DE, et al. (2019) Diabetic neuropathy. Nat Rev Dis Primers 5(1): 41.
- SBD (2020) Guidelines of the Brazilian Society of Diabetes 2020. Brazil.
- Markman B, Carlos A, Sousa S, Felice A, Issa C, et al. (2021) Guidelines Brazilian on Hypertension-2020 Guidelines. 116: 516-658.
- Leitzmann MF, Park Y, Blair A, Barbash RB, Mouw T, et al. (2007) Physical activity recommendations and decreased risk of mortality. Arch Intern Med 167(22): 2453-2460.
- Zagury RL (2016) Handbook on exercise for people with type 2 diabetes.
- Cândida M, Parisi R, Raquel C, Leite M, Fabrício M, et al. (2020) Interdisciplinarity in the context of the diabetic foot: Clinical treatments, public policies and health technologies.
- Martins IJ (2016) Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Adv Aging Res 5(1): 9-26.
- James MI (2019) Insulin therapy and autoimmune disease with relevance to non alchoholic fatty liver disease. In: Nonalcoholic Fatty Liver Disease-An Update. IntechOpen.
- Hussey SE, McGee SL, Garnham A, Wentworth JM, Hargreaves M (2011) Exercise increases skeletal muscle GLUT4 gene expression in patients with type 2 diabetes. Diabetes Obes Metab 14(8): 768-771.
- Oliveira BBB, Viana NS, Pereira WVC, da Silva AN (2015) Comparison of capilary glycemic responses after moderated continuous racing and high-intensity interval training in diabetes type 1 patients. Diabetol Metab Syndr 7: A228.
- Yardley JE, Kenny GP, Perkins BA, Riddell MC, Malcolm J, et al. (2012) Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care 35(4): 669-675.
- Guelfi KJ, Jones TW, Fournier PA (2005) The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care 28(6): 1289-1294.
- Bussau V, Ferreira L, Jones T, Fournier P (2006) The 10-s maximal sprint: A novel approach to counter an exercise-mediated fall in glycemia in individuals with type 1 diabetes. Diabetes Care 29(3): 601-606.
- Eicher JD, Maresh CM, Tsongalis GJ, Thompson PD, Pescatello LS (2010) The additive blood pressure lowering effects of exercise intensity on post-exercise hypotension. Am Heart J 160(3): 513-520.
- Santos LP, Moraes RS, Vieira PJC, Ash GI, Waclawovsky G, et al. (2016) Effects of aerobic exercise intensity on ambulatory blood pressure and vascular responses in resistant hypertension: A crossover trial. J Hypertens 34(7): 1317-1324.
© 2021 Campos pereira WV. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






