- Submissions

Full Text
Intervention in Obesity & Diabetes
Managing Type 1 Diabetes from Gynecological Waste: Trash to Treasure
Kumbhar RG and Desai S*
Department of Pharmacology, Dr. D Y Patil Institute of Pharmaceutical Sciences and Research, Pune
*Corresponding author:Shivani Desai, Department of Pharmacology, Dr. D Y Patil Institute of Pharmaceutical Sciences and Research, Sant Tukaram Nagar, Pimpri, Pune (Maharashtra)-411018, India
Submission:June 22, 2021;Published: August 18, 2021

ISSN 2578-0263Volume5 Issue3
Abstract
Type 1 Diabetes Mellitus (T1D) is an autoimmune disease that destroys β cells. β cells play a critical role in glucose homeostasis by sensing blood glucose and releasing insulin to maintain physiologic glucose levels within a relatively narrow range. Human UCB cells pose a lower risk of viral contamination due to the low placental transmission rates during prenatal life. They have superiorities including low immunogenicity, non-invasive harvest procedure, gynecological waste, easy expansion in vitro, and ethical access compared with stem cells from other sources. Based on available preclinical data and therefore the agreement that infusion of minimally manipulated autologous cord blood cells was likely to be extremely safe. Thus, the UCB-derived stem cells transplant does not require a perfect match of MHC as bone marrow transplants do. Depending on the degree of differentiation, the ability to regenerate themselves, and the origin of many stem cell types can be differentiated. In terms of their plasticity totipotent, pluripotent, multipotent and weak stem cells are present. The use of Stem Cells (SCs) holds great promise for the cure of T1D due to their propitious immunological characteristics and their regenerative capabilities. Because it is free from ethical complications and straightforward to isolate without invasive methods, human Umbilical Cord Blood (hUCB) has become a precious medical product.
Keywords:Type 1 diabetes mellitus; Umbilical cord blood stem cells; Immunogenicity; Glucose homeostasis; Gynecological waste; Plasticity
Abbreviations: T1D: Type 1 Diabetes Mellitus; GVHD: Graft-Versus-Host Disease; AD-MSCs: Adipose Tissue-Derived Mesenchymal Stem Cells; MHC: Major Histocompatibility Complex
Introduction
Historically, type 1 diabetes has been considered a disorder in children and adolescents, but this perception has changed over the past decade, so age at onset of symptoms is no longer a barrier. Polydipsia, polyphagia, and polyuria (early trio of symptoms associated with the onset of the disease) and apparent hyperglycemia are always diagnostic symptoms in children and adolescents, and at a lower rate in adults [1]. Type 1 Diabetes Mellitus (T1DM) is generally considered to have an autoimmune etiology, which occurs as a result of the destruction of irreversible and specialized insulin-producing cells that produce pancreatic β cells in the islets of Langerhans [1,2]. Likewise, there is an urgent need to develop new therapies that will help not only to manage the disease, but also hopefully provide a real cure for DM [3]. One potential therapy for diabetic patients is infusion of donor islets of Langerhans into the hepatic portal vein. In this procedure, islets containing the insulin secreting b cells are transplanted from a cadaveric donor to the patient. This procedure has been found to be effective in removing T1DM patients from insulin treatment to some degree. However, there is a variation in the quality of donor cells that continues to lead to a split in the success of the results [4]. β cells play a critical role in glucose homeostasis by sensing blood glucose and releasing insulin to maintain physiologic glucose levels within a relatively narrow range [2]. The blood of the umbilical cord is filled with T cells (Tregs) and many types of stem cells, which show the ability to detoxify, and hold promise in their ability to restore the tolerance associated with pancreatic islet cells through the correction of immune responses and compression T cells. Recently, the use of autologous umbilical cord blood or body cells from cord blood has been suggested as a new treatment for T1DM, with less harmful benefits to donors, less behavioral anxiety, lower incidence of Graft-Versus-Host Disease (GVHD) and more readily available [5]. Type 1 Diabetes Mellitus (T1D) is an autoimmune disease that destroys β cells. As a result, insulin secretion and the regulation of glucose levels in the blood are impaired. The onset of the disease usually occurs between 6 and 12 years of age [6]. About 382 million people worldwide are affected by diabetes; the rate is expected to increase to 592 million by 2035 [7]. World Health Organization (WHO) and American diabetes association has classified T1DM immune-mediated and idiopathic disease. In diabetic patients, glycosylated hemoglobin and blood sugar levels help to determine diabetes while low levels of serum C-peptide serve as the most acceptable indicator of T1DM diagnosis [8]. The disease is believed to be autoimmune in nature and triggered by several factors like seasons of autumn and winter, viral/bacterial infections and environmental pollutants [9]. The defective insulin release characteristic of T1DM reflects progressive β cell destruction within the range of 60-100%, counting on disease duration 4-7, also as functional defects (for example, defective glucose-induced insulin release and delayed conversion of proinsulin to insulin) that are probably caused by local release of proinflammatory mediators by infiltrating immune cells. Pancreatic pathology among patients with T1DM is different, with varying degrees of insulitis and cell loss seen in different lobes of the pancreas [10]. Patients with high b-cell mass initially experience less microvascular complications and less hypoglycemic events than those of patients with high b-cell mass. In face of this, b-cell preservation is another important goal in the management of type 1 diabetes and related complications [11]. The microbiome, thought about the “second genome” of the host, is changed over into Type 1 Diabetes Mellitus (T1DM) by patients with dysbiosis. Dysbiotic gut microbiota can reduce MSC treatment, and moderation of gut microbiota can help improve the results of MSC transplantation. Minor immune to intestinal dysbiosis of the gut has been suggested as an immunosuppressant for T1DM. In addition, dysbiosis of the gut microbiota can also affect the function of the intestinal barrier. It has been suggested that dysfunction of the intestinal tract in T1DM may increase the presence of gut microbiota in systemic proliferation and, in turn, cause islet cell dysfunction [12].
Stem Cells
Stem cells can be prepared from embryonic or adult stem cells. However, the use of embryonic stem cells is controversial since the accumulation of such cells is achieved by killing embryos. For this reason, embryonic stem cell accession or possession is restricted in most states or excluded from public funds [13]. The autoimmune destruction of insulin producing cells that characterizes this disease requires a constant supply of insulin to maintain normal glycemic level. The shortage of transplantable human islets have evoked a large-scale interest in probing stem cells as an alternative sources of cells [14]. They can be derived from many types of tissues and organs such as bone marrow, umbilical cord, placenta, cartilage, and adipose tissue [15]. The use of Stem Cells (SCs) holds great promise for the cure of T1D due to their propitious immunological characteristics and their regenerative capabilities [16]. The immunomodulation properties of stem cells are often helpful to regulate a balance between β-cell destruction and their regeneration. Mouse and human hepatic stem cells were differentiated into insulin-secreting β-like cells and used to overcome the condition of hyperglycemia. BM-MSCs are additionally ready for graft acceptance and diminish autoimmunity [17]. There are small numbers of stem cells in the human body that after division with mitosis can differentiate into daughter cells or create newer stem cells. Maintenance and activation of their differentiation potential is fundamentally influenced by the microenvironment (cellular and humoral). Depending on the degree of differentiation, the ability to regenerate themselves, and the origin of many stem cell types can be differentiated. In terms of their plasticity totipotent, pluripotent, multipotent and weak stem cells are present. Large cells (e.g., zygote, spore, or morula) can form any human cell or even the entire body. In the case of pluripotent cells, the chances of forming a complete functional organization are limited. Many stem cells are able to create specific types of daughter cells. Under physical conditions, they ensure continuous tissue regeneration, replace dead somatic cells, and after injury participate in regeneration of the affected organ (Figure 1). Unipotent cells are precursor/progenitor cells with limited plasticity [18]. In recent years, Mesenchymal Stem Cells (MSCs) have been highlighted as a novel regenerative therapy for their multipotency, self-renewal, low immunogenicity and high immunomodulatory properties. Exosomes derived from Adipose Tissue-Derived Mesenchymal Stem Cells (AD-MSCs) have immunomodulatory effects of T-cell inflammatory response and reduction of clinical symptoms on streptozotocin-induced of the Type-1 Diabetes Mellitus (T1DM). Since transplantation of multipotent stem cells renders potential risks, secretome derivatives of these cells may be considered as promising practical therapeutics in regenerative medicine for treatment of T1DM [19]. An effective method is to save the remaining cells, restore cell function, and protect the replace IPCs from autodestruction. Pluripotent stem cells, including Embryonic Stem (ES) and induced Pluripotent Stem (iPS) cells offer a valuable alternative to supply the required cells to substitute organ transplants but also serve as a model for learning the onset and progression of the disease, resulting in better treatments [20]. Three intraperitoneal injections of ADSCs can reverse hyperglycemia in 78% of diabetic NOD mice, characterized by increased insulin, amylin, and glucagon like peptide 1 levels. Early onset diabetes recovery occurs by blocking the immune response that protects CD4+ Th1 and improving regulatory T-cells near lymph nodes. These results are the first direct evidence for ADSCs in balancing the autoimmune response, while MSCs have been widely investigated for their immunomodulatory properties in cellular transplantation [21].
Figure 1: Umbilical cord blood stem cell applications.
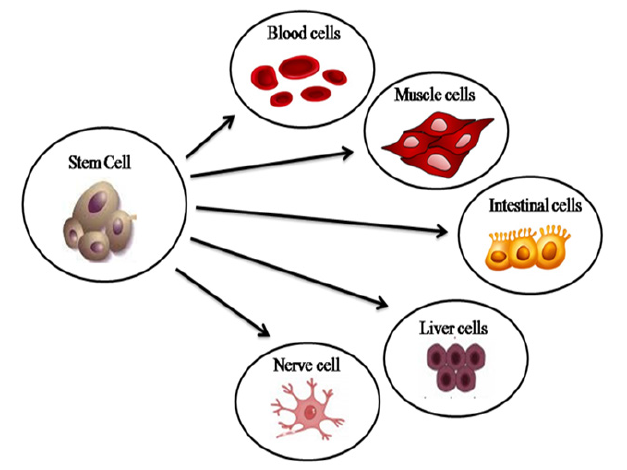
Umbilical Cord Blood Stem Cell
UCB is preferable due to its immediate availability, absence of
risk to the donor (and, if autologous, to the recipient as well) low
risk of graft-vs-host disease, and increased capacity for expansion
The ability of UCB-derived stem cells to differentiate into a variety
of non-blood cell types, including hepatocytes, neural cells,
and endothelial cells has already been documented. They have
superiorities including low immunogenicity, non-invasive harvest
procedure, gynecological waste, easy expansion in vitro, and ethical
access compared with stem cells from other sources. Therefore,
HUC-MSCs can be a promising target for cell-based therapy [22]. As
further evidence of the potential use of UCB in T1D therapies, UCB
stem cells have successfully been directed in vitro to differentiate
into insulin and c-peptide–producing cells. Because cord blood
contains an outsized population of immature unprimed highly
functional regulatory T lymphocytes, this might be the foremost
important reason for exploring therapeutic applications of UCB in
T1D. The number of highly active T cells in UCB can work to reduce
the inflammatory response of cytokines and increase the ability
of active T cells, which play an important role in the cellular selfregulatory
process [23].
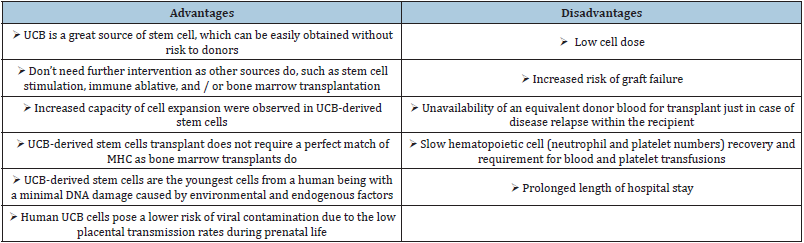
I. UCB is an abundant stem cell source, which can be easily
obtained with no risk to the donors;
II. They do not require additional intervention, such as stem
cell stimulation, immune ablative, bone marrow transplantation
III. Increase in cell proliferation
IV. Due to the low expression level of Major Histocompatibility
Complex (MHC) antigens and costimulatory molecules,
UCB-derived stem cell can easily escape from the immune
recognition by the recipient lymphocytes and will not induce the
proliferation of allogeneic lymphocytes after transplantation.
Thus, the UCB-derived stem cells transplant does not require a
perfect match of MHC as bone marrow transplants do.
V. UCB-derived stem cells are the youngest cells from
a human being with a minimal DNA damage caused by
environmental and endogenous factors.
VI. Human UCB cells pose a lower risk of viral contamination
due to the low placental transmission rates during prenatal life
[5].
The Umbilical Cord (UCB) plays a vital role in the transport
of nutrients and oxygen between mother and fetus. Because it
has no ethical issues and is easy to diagnose without invasive
methods, human Umbilical Cord (hUCB) has become a valuable
medical product [24]. Umbilical cord blood is rich in Tregs, which
activates and suppresses antigen regeneration, and is also a source
of haematopoietic cells and pluripotent stem. Therefore, there is a
strong scientific reason behind cord blood to prevent or delay the
onset of type 1 diabetes [25]. Cells derived from umbilical cord
blood may represent an important biological resource for β cell
generation, as well as a source of immune-modulatory cells for T1DM therapy. Human umbilical cord blood has great potential as a
rich source of stem cells, including UCB-MSCs and UCB-SCs, which
can be readily isolated from umbilical cord blood without ethical
problems (Table 1). The properties of weak immunogenicity and
strong immunoregulation make these cells promising for treating
T1DM [24]. The cord blood for preservation and subsequent use is
the one that is obtained from the umbilical cord after birth. Although,
the HUCB was discarded in the past, recent studies have shown that
it is a rich source of stem cells that are therapeutically useful in
many malignant and nonmalignant diseases. Consequently, HUCB
stays a choice to bone marrow transplantation in clinical practice
[26]. Based on available preclinical data and the agreement that
infusion of minimally manipulated autologous cord blood cells was
likely to be extremely safe [23].
Table 1: Advantages and disadvantages of human umbilical cord blood.
Conclusion
Cells taken from the umbilical cord blood may indicate an important cell for β cell generation, as well as a source of antibodies to T1DM treatment. The characteristics of weak immunogenicity and strong immunity make these cells promising to treat T1DM. In conclusion, with promising data from preclinical studies and clinical studies, therapeutic implants of blood-derived umbilical cord cells, whether UCBMSC or UCB-SCs, can oversee the safe and effective treatment of T1DM patients.
Acknowledgement
The authors are thankful to the Principal, Dr. Sohan Chitlange, and the management of Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pune. We are also thankful to Dr. D. Y. Patil Vidyapeeth, Pune, for extending continuous support and guidance to write this paper.
References
- Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383(9911): 69-82.
- Bluestone JA, Herold K, Eisenbarth G (2010) Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464(7293): 1293-1300.
- Kassem DH, Kamal MM (2020) Therapeutic efficacy of umbilical cord-derived stem cells for diabetes mellitus: A meta-analysis study. Stem Cell Res Ther 11(1): 484.
- Kakkar A, Sorout A, Tiwari M, Shrivastava P, Meena P, et al. (2018) Current status of stem cell treatment for type 1 diabetes mellitus. Tissue Eng Regen Med 15(6): 699-709.
- He B, Li X, Yu H, Zhou Z (2015) Therapeutic potential of umbilical cord blood cells for type 1 diabetes mellitus. J Diabetes 7(6): 762-773.
- Bandeiras C, Hwa AJ, Cabral JMS, Ferreira FC, Finkelstein SN, et al. (2019) Economics of beta-cell replacement therapy. Curr Diab Rep 19(9): 75.
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, et al. (2013) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103(2): 137-149.
- Farooq T, Rehman K, Hameed A, Akash MSH (2019) Stem cell therapy and type 1 diabetes mellitus: Treatment strategies and future perspectives. Adv Exp Med Biol 1084: 95-107.
- Vanikar AV, Trivedi HL, Thakkar UG (2016) Stem cell therapy emerging as the key player in treating type 1 diabetes mellitus. Cytotherapy 18(9): 1077-1086.
- De Beeck AO, Eizirik DL (2016) Viral infections in type 1 diabetes mellitus-why the β cells? Nat Rev Endocrinol 12(5): 263-273.
- Couri CEB, Voltarelli JC (2008) Autologous stem cell transplantation for early type 1 diabetes mellitus. Autoimmunity 41(8): 666-672.
- Lv W, Graves DT, He L, Shi Y, Deng X, et al. (2020) Depletion of the diabetic gut microbiota resistance enhances stem cells therapy in type 1 diabetes mellitus. Theranostics 10(14): 6500-6516.
- Katuchova J, Harvanova D, Spakova T, Kalanin R, Farkas D, et al. (2015) Mesenchymal stem cells in the treatment of type 1 diabetes mellitus. Endocr Pathol 26(2): 95-103.
- Burrack AL, Martinov T, Fife BT, Fife BT (2017) T cell-mediated beta cell destruction: Autoimmunity and alloimmunity in the context of type 1 diabetes. Front Endocrinol (Lausanne) 8: 343.
- Marrow B (2012) Concise review: Mesenchymal stem cells for diabetes. Stem Cells Transl Med 1(1): 59-63.
- Fiorina P, Voltarelli J, Zavazava N (2011) Immunological applications of stem cells in type 1 diabetes. Endocr Rev 32(6): 725-754.
- Peng B, Dubey NK, Mishra VK, Tsai F, Dubey R, et al. (2018) Addressing stem cell therapeutic approaches in pathobiology of diabetes and its complications. J Diabetes Res 2018: 7806435.
- Műzes G, Sipos F (2019) Issues and opportunities of stem cell therapy in autoimmune diseases. World Journal of Stem Cells 11(4): 212-221.
- Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, et al. (2018) Immunomodulatory effects of mesenchymal stem cell – derived exosomes on experimental type ‐ 1 autoimmune diabetes. J Cell Biochem 119(11): 9433-9443.
- Schroeder IS (2012) Potential of pluripotent stem cells for diabetes therapy. Curr Diab Rep 12(5): 490-498.
- Lin H, Chan T, Fu R, Chuu C, Chiu S, et al. (2015) Applicability of adipose-derived stem cells in type 1 diabetes mellitus. Cell Transplant 24(3): 521-532.
- Li T, Xia M, Gao Y, Chen Y, Xu Y (2015) Human umbilical cord mesenchymal stem cells : An overview of their potential in cell-based therapy. Expert Opin Biol Ther 15(9): 1293-1306.
- Haller MJ, Viener HL, Wasserfall C, Brusko T, Atkinson MA, et al. (2008) Autologous umbilical cord blood infusion for type 1 diabetes. Exp Hematol 36(6): 710-715.
- Stiner R, Alexander M, Liu G, Liao W, Liu Y, et al. (2019) Transplantation of stem cells from umbilical cord blood as therapy for type I diabetes. Cell Tissue Res 378(2): 155-162.
- Han MX, Craig ME (2013) Research using autologous cord blood - time for a policy change. Med J Aust 199(4): 288-290.
- Reddi AS, Kuppasani K, Ende N (2010) Human umbilical cord blood as an emerging stem cell therapy for diabetes mellitus. Curr Stem Cell Res Ther 5(4): 356-361.
© 2021 Desai S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






