- Submissions

Full Text
Intervention in Obesity & Diabetes
Importance of Antidiabetic Medicinal Plants in the Prevention and Management of Insulin Resistance: Case of Gestational Diabetes in Cameroon
Nolé T1* and Wilfried Lionel TD2
1Higher Institute of Environmental Sciences, Cameroon
2Centre de Cardiologie et Medical, Yaounde, Cameroon
*Corresponding author:Tsabang Nolé, Higher Institute of Environmental Sciences, Yaounde, Cameroon
Submission:April 01, 2021;Published: May 05, 2021

ISSN 2578-0263Volume5 Issue2
Abstract
During pregnancy, insulin resistance increase over time. So in the last four months of the pregnancy, insulin resistance upsurges significantly and can become severe, specifically in women with gestational diabetes and type 2 diabetes. Various factors including placental hormones, obesity, and inactivity, an unhealthy nourishment, and inherited and epigenetic influences negatively impacts insulin resistance in pregnancy, but the causal mechanisms are multifaceted and still not completely elucidated. Therefore in this review, we seek to identify among antidiabetic plants usually used in Cameroon those which can regulate insulin resistance in pregnant women, with diabetes. To achieve this objective a literature search was performed in Google, Google Scholar and PubMed, using the key “plants improving insulin sensitivity or insulin resistance in gestational diabetes”. The phytochemicals with insulin resistance and insulin sensitivity of recorded antidiabetic plants and their action mechanisms were compared with those of antidiabetic plants regularly used In Cameroon. The phytochemicals and their active mechanisms were searched using the keys «what is a given compound) chemical group(s). A list of Cameroonian known antidiabetic plants improving insulin sensitivity and insulin resistance were established. Nineteen (19) antidiabetic medicinal plants usually castoff in Cameroon have been reported to have a beneficial effect on insulin sensitivity and insulin resistance with regard to the presence of molecules which improve insulin sensitivity and insulin resistance in their chemical composition. Finally, well-chemical study, well mechanisms elucidation and well-designed randomized controlled trials with lasting consumption are still required to assure the bioactivity and safety of these medicinal plants and compounds for gestational diabetic patients.
Keywords:Type 1; Type 2 diabetes mellitus; Gestational diabetes; Antidiabetic activity; Herbal medicines; Insulin insensitivity; Previous mechanisms of action
Abbreviations: PCOS: Polycystic Ovary Syndrome; BMI: Body Mass Index; HCG: Human Chorionic Gonadotropin; HPL: Human Placental Lactogen; HPCH: Human Placental Growth Hormone; InsR-α: Insulin Receptor α; IRS-1: Insulin Receptor Substrate-1; PI3K: Phosphatidylinositol 3-Kinase; AMPK: Adenosine Monophosphate Activated Protein Kinase; ACC: Acetyl-CoA Carboxylase; MAPKs: Mitogen-Activated Protein Kinases; PPARγ: Peroxisome Proliferator-Activated Receptors; GLUT: Glucose Transporter; PTP1B: Protein Tyrosine Phosphatase 1B; TNF: Tumor Necrosis Factor; TZDs: Thiazolidinediones; CAMP: Cyclic Adenosine Monophosphate; SIRT1 (Sirtuin 1): Silent Information Regulator 1; NAFLD: Non-Alcoholic Fatty Liver Disease; LPS: Lipopolysaccharide; ALI: Acute Lung Injury; NO: Nitric Oxide: MODS: Multiple Organ Dysfunction Syndrome; CR: Calorie or Dietary Restriction, SIRT2: Silent Information Regulator 2
Introduction
Gestational diabetes mellitus is the most common metabolic disorder during pregnancy with health consequences for both mother and progenies lives during and after pregnancy. Insulin resistance is the shrinkage in the biological response to a given insulin dose, whether endogenous or exogenous, in the target tissue including liver, muscle or adipose tissue [1]. Obesity is an essential cause of insulin resistance, and the changes in insulin indifference through pregnancy are partly related to mother fat mass [2]. The physiology of insulin resistance during pregnancy is captivating and limits glucose utilization in pregnant women. The important of glucose is thereby oriented to supply the growing of fetus, which requests glucose as most of its energy source. Henceforth, the degree of insulin resistance in pregnant women is proportional to the degree of glucose fluctuation from the mother to the fetus [2,3].
Obesity
THyperglycemia during pregnancy proves fetal hyperglycemia and hyperinsulinemia, principal causes of fetal macrosomia, which are the most common and thoughtful complications of gestational diabetes and obesity. Obesity among women in the reproductive age has been increased, during the last decades. The deterioration in the physiologic insulin resistance is the main consequence of this obesity that creates a negative impact on the intrauterine atmosphere, which affects perinatal program design and metabolic dysfunction in the progenies. Obesity and overweight are significantly involved in the declining natural fertility and decrease the effectiveness of treatments. Women with obesity and/or Polycystic Ovary Syndrome (PCOS) have abnormal plasma adiponectin and resisting profiles. In addition, the Body Mass Index (BMI) has a larger impact on insulin resistance in women with PCOS in general compared to healthy controls [4]. In pregnancy, PCOS is associated with a higher gestational weight gain and accordingly exacerbated pregnancy and infant outcomes [5].
Origin and role of adiponectin
TAdiponectin is a protein hormone, which is involved in a number of metabolic processes, like regulating glucose levels and fatty acid oxidation. In humans it is secreted from mostly adipose tissue, but also in muscle, and even in the brain and also from the placenta in pregnancy into the bloodstream. Adipokines such as adiponectin and resisting modulate steroidogenesis of gonadic somatic cells, germ cell maturation and secretion of gonadotrope hormones in various species. The reproductive system is tightly coupled with energy balance, and thereby metabolic abnormalities can lead to the development of physio pathological situations such as the Polycystic Ovary Syndrome (PCOS). Thus, these adipokines could be a link between reproduction and energy metabolism and could partly explain some infertility related to obesity or PCOS. Many studies have found adiponectin to be inversely correlated with body mass index in patient populations. Moreover, Adiponectin circulating levels are decreased in individuals with obesity, atherosclerosis and insulin resistance, suggesting that its deficiency may have a causal role in the etiopathogenesis of these diseases [6]. Studies have shown that adiponectin administration has insulin-sensitizing effect in type 2 diabetes and many studies have demonstrated a significant inverse association between adiponectin) and insulin resistance [6,7]. Finally, uncoupling of oxidative phosphorylation represents a new alternative to inhibition for triggering cytoprotective effects of therapeutic relevance to insulin resistance in both muscle and liver [8].
Development of insulin resistance in gestational diabetes
TIn a normal pregnancy, mother tissues become gradually unresponsive to insulin. An important diminution in insulin sensitivity is advancing for gestation both in women with normal glucose tolerance and in women with gestational diabetes [9]. In the first women group, the changes in insulin sensitivity are overcome by a sufficient increase in insulin secretion by pancreatic beta cells, but in second group, endogenous insulin discharge is inadequate during pregnancy [9]. In type 1 diabetic women insulin requirements growth usually by 70% during pregnancy [10]. Between gestational week 11 and week 16, there is a minor decrease in insulin requirements. This is a consequence of an improvement in insulin sensitivity that is known to upsurge the risk of especially nighttime hypoglycemia in type 1 diabetic women. From week 20, there is a substantial intensification in insulin requirements as a result of an obvious reduction in insulin sensitivity until week 33 [9]. Type 2 diabetic pregnant women require a much greater increase in insulin dose from the start to the end of each trimester, and insulin requirements do not decline in early and late pregnancy as is the case in women with type 1 diabetes [11,12].
Role of placenta
The persistent insulin resistance in gestational diabetes may be related to inflammatory factors, mediated by action of placental hormones and other cytokines, affecting the post receptor insulin signaling cascade [3,13]. The increase in the rises of free fatty acids and lip toxicity, the combination of excess lipid and glucose supplies to the fetus and a suboptimal placental function and metabolic environment in utero consequently increase the risk of metabolic disease in the progenies like inflammation, endothelial dysfunction, a decrease in trophoblast invasion, and consequently a reduction in placental metabolism and function [14,15]. The placenta certainly plays a critical role in the development of insulin resistance in pregnant women. Consequently, it is extraordinary that there is a typical speedy repair of glucose homeostasis instantaneously after the ejection of the placenta at delivery. Thus placenta is placed as the interface between the mother and fetus. So the alterations of placenta structure and it function may impact fetal growth and development. The interchange of glucose between a mother and a fetus is pivotal for fetal growth and wellbeing. Outstanding to the important role of the placenta and the glucose metabolism herein, it eagerly influences the complications of gestational diabetes like toxic effects of insulin resistance and circulating insulin levels on placental tissues, at least in early pregnancy [16]. The placenta discharges pregnancy-specific hormones like estradiol, progesterone, prolactin, cortisol, Human Placental Lactogen (hPL), eptin, Human Chorionic Gonadotropin (HCG) and hPGH into the mother circulation. These hormones have previously been described to be mediators of the change in insulin sensitivity during gestation. Thus, at present, no single hormone has been found to explain the insulin resistance of pregnancy [17]. Therefore the causes and contributory mechanisms of insulin resistance in pregnancy are multifaceted and still not completely discovered. But in Cameroon, it was observed that many women who used antidiabetic plants published by our team, during pregnancy, ameliorate their condition. Therefore in this review, we seek to identify among these plants those which can regulate insulin resistance in pregnant women. Do antidiabetic plants play an important role in the control of the hormonal and metabolic factors that have been described to determine the development of insulin resistance in human pregnancy until now?
Methodology
A literature search was performed using two keys “plants
improving insulin sensitivity in gestational diabetes” and sirt1
activation properties by any recorded plant used in Cameroon for
gestational diabetes. The 2 keys permitted to identify 74 articles.
Species involved in this work are adequate nourishment, coadjutant
therapies such as medicinal plants popularly used to reduce
diabetes-induced hyperglycemia, within the context of pregnancy,
with safety for the mother and fetus and utilized in Cameroon. The
animal models encountered in this study include:
a. Rats fed high-fructose diet;
b. Diabetic rats and 3T3-L1cells;
c. Streptozotocin-induced type 2 diabetic rats;
d. L8 muscle cells;
e. High-fat diet mice;
f. Insulin resistant mice;
g. and randomized controlled trial in type 2 diabetic patients
A literature search was performed in Google, Google Scholar
and PubMed, using the key “plants improving insulin sensitivity
or insulin resistance in gestational diabetes”. The antidiabetic
phytochemicals and their active mechanisms were searched using
the keys “what is a given antidiabetic compound) chemical group(s)”.
To know more the chemical groups of recorded phytochemicals,
research were performed using the keys “(what is chemical group
(s) of an identified antidiabetic compound)”. The confirmation of
the anti-diabetic effects of plants used in Cameroon by patients with
gestational diabetes was established on the basis of the presence
in these plants of confirmed anti-diabetic chemical compounds
isolated in anti-diabetic plants identified in the literature on the one
hand or by direct bibliographic research on these plants on the other
hand. A list of Cameroonian known antidiabetic plants improving
insulin sensitivity and insulin resistance were established. The
plants authentication was performed from the Project of the World
Flora Online Consortium and at National herbarium of Cameroon.
Results
Antidiabetic plants improving insulin sensitivity
Nineteen (19) antidiabetic medicinal plants usually castoff in
Cameroon have been reported to have a beneficial effect on insulin
sensitivity and insulin resistance with regard to the presence of
molecules which improve insulin sensitivity and insulin resistance
in their chemical composition. Some medicinal plants reported
in this review revealed the improvement of insulin sensitivity by
regulating adiponectin levels [18,19]. These medicinal plants with
beneficial action on insulin sensitivity act via various cellular and
metabolic targets. The principle sites targeted by these medicinal
plants are the peripheral tissues (muscles and adipocytes) or
the liver. In these organs, these medicinal plants have been
demonstrated to stimulate:
a. Insulin Signaling (the expression levels of insulin
receptor α subunit (InsR-α), Insulin Receptor Substrate-1
(IRS-1), Phosphatidylinositol 3-Kinase (PI3K), tyrosine
induced phosphorylation of insulin receptor substrate, the
phosphorylation of Adenosine Monophosphate Activated
Protein Kinase (AMPK)/ Acetyl-Coa Carboxylase (ACC) and
Mitogen-Activated Protein Kinases (MAPKs);
b. PPARγ;
c. Glucose transport by enhancing the glucose uptake and
the expression of the GLUT4 as well as its translocation and
Glucose Transporter 2 (GLUT2);
d. Activity of hexokinase, phosphofructokinase, pyruvate
kinase, glucose-6-phosphatase, fructose-1,6-bisphosphatase
and glucose-6-phosphate dehydrogenase, liver glucokinase and
liver glycogen content;
e. Gene expression of resisting
In addition, several medicinal plants have reported to inhibit:
i. Activity of PTP1B
ii. Hepatic glucose production
iii. Tyrosine dephosphorylation
iv. Hepatic gluconeogenesis
v. Serum levels of leptin
vi. TNF-α [20]
All the cited cellular and metabolic targets up cited are well
documented such as the role of insulin- signaling pathways, the
glucose transport and metabolism in the regulation of glucose
homeostasis [20-28]. PPARγ plays a critical role in glucose
homeostasis and serves as the molecular target for a class of
insulin-sensitizing drugs called TZDs. TZDs are PPARγ ligands
and widely used for treatment of type 2 diabetes, they had very
minimal activity toward PPAR-α or PPAR-β. Effects on insulin action
in tissues would occur as a consequence of alterations in signaling
molecules produced by fat, such as free fatty acids, TNF-α, leptin
or others [29]. PPARs are dietary lipid sensors that control energy
homeostasis and playing an important role in the management
of metabolic disorders, such as type 2 diabetes, hyperlipidemia,
insulin resistance and cardiovascular diseases [30].
In this review, several medicinal plants are reported to modulate
PPARs and then improving insulin sensitivity. However, for all
antidiabetic plants reported in this review, a general remark had
been noticed about the safety indicating that a little information is
available in these studies concerning the toxicological studies. In
the other hand, few studies have performed advanced purification
determining some phytochemical groups involved in improving
insulin sensitivity [31]. The main phytochemical groups improving
insulin sensitivity, their chemical structure and action mechanisms
of plants recorded are:
Flavonoids: Flavonoids are a group of natural constituents with variable phenolic structures and are found in plants (Figure 1), [32]. Ocimum gratissimum L (Lamiaceae) contains flavonoids and phenols (Figure 2). These phytoconstituents and the leaves aqueous extract significantly decreased the blood glucose level after 24h of administration in streptozocin-induced diabetic rats and showed at the dose of 500mg/kg, a significant reduction in fasting blood glucose level in diabetic pregnant rats and in nondiabetic pregnant rats [33].
Figure 1: Basic flavonoid structure [32].
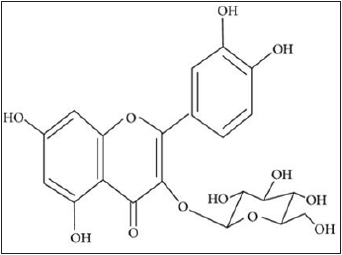
Figure 2: Structure of important terpenes of each class [33].
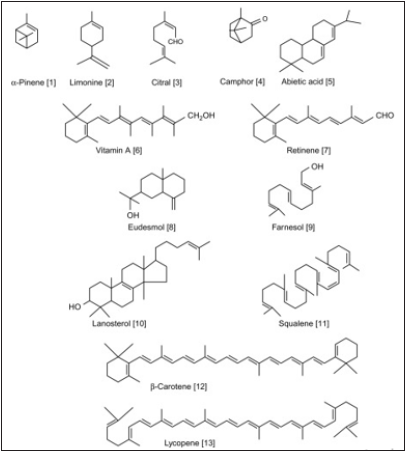
Terpenoids: Terpenoids, sometimes called isoprenoids, are a large and varied class of naturally occurring organic chemicals derivative from the 5-carbon compound isoprene, and the isoprene polymers called terpenes. Most are multicyclic structures with oxygen-containing functional groups (Figure 3). Terpenes are hydrocarbons. Depending upon the number of isoprene units, terpenoids are classified as in (Figure): [34]. The pharmacological action of Panax ginseng C.A. Mey (Araliaceae) has been attributed to the ginsenosides, a group of triterpenoid saponins. Certainly, more than 150 naturally happening ginsenosides have been found in the roots, leaves, stems, fruit, and flower heads of innumerable Panax species. Numerous clinical trials and animal studies have established that ginseng and ginsenosides increase insulin sensitivity and control lipid metabolic [35].
Figure 3: Structures of glycosides [34].
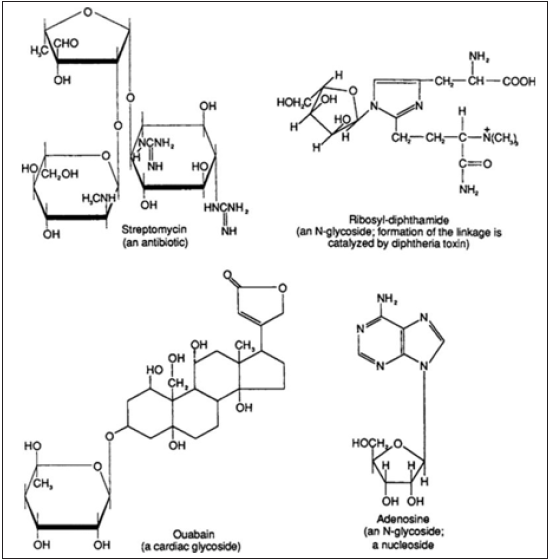
Glycosides: In correct terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond (Figure 4). The sugar group is then known as the glycone and the non-sugar group as the aglycone or genin part of the glycoside [34]. Quercetin and quercetin 3-O-glycosides isolated from Holarrhena antidysenterica (L.) Wall (Apocynaceae) were found to have potential applications for the prevention and treatment of insulin resistance in C2C12 muscle cells by motivating glucose uptake and the AMPK pathway [36].
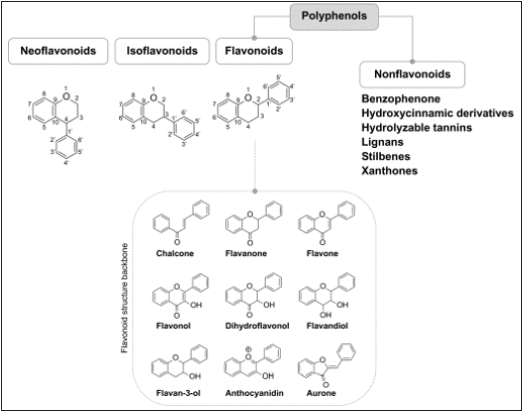
Polyphenols: are a group of chemical constituents isolated from plants that are characterized by the presence of more than one phenol unit or building block per molecule [36]. The polyphenolics found in Bulbine abyssinica A. Rich, (Asphodelaceae) leaves have substantial antioxidant properties and inhibitory effects against α-amylase and α-glucosidase enzymes. The antidiabetic activities could be due to the carvone, quercetin, and psoralen isolated from the plant [35].
Figure 4: Chemical classification of polyphenols [36].
Aloin: which is an anthraquinone glycosyl, meaning that its anthraquinone skeleton has been modified by the addition of a molecule of sugar (Figure 5), [37].
Figure 5: Chemical structures of aloin [38].
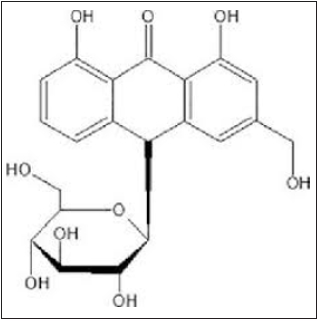
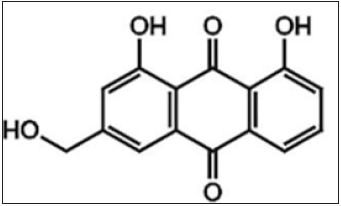
Aloe emodin: which is a dihydroxyanthraquinone that is chrysazin carrying a hydroxymethyl group at position 3. Aloin and aloe-emodin seem to be involved in antidiabetic effect (Figure 6). The central role of the nuclear receptor PPARγ in lipid and glucose metabolism is well established; however, due to some adversative effects numerous PPARγ-targeting drugs have limited beneficial effect [38]. A polyphenol-rich Aloe spp (Xanthorrhoeaceae) extract containing both aloin and aloe-emodin was able to reduce significantly body weight and blood glucose levels and protected animals against disapproving results on insulin resistance when administered orally for a period of 4 weeks to insulin-resistant mice [39]. In a clinical study, an Aloe vera gel complex has been demonstrated to reduce the fasting blood glucose, body weight and insulin resistance in obese individuals with prediabetes or premature diabetes mellitus [40].
Figure 6: Chemical structures of aloe emodin [39].
Xanthones like Mangiferin, also known as alpizarin or chinomin. Mangiferin is a xanthone compound isolated from Anemarrhena asphodeloides Bunge (Asparagaceae) rhizome (Figure 7). Tested for antidiabetic properties in KK-Ay mice, of diabetes type-2, it dropped significantly the blood glucose concentration of this animal model 3 weeks after oral ingestion [41]. Nevertheless, no effect on the blood glucose level in normal mice was seen, indicating that Mangiferin could be useful in managing type-2 diabetes (Figure 8). Therewith, Mangiferin improved hyperinsulinemia and, on insulin tolerance test, reduced blood glucose concentration of KK-Ay mice. From these findings, it seems possible that Mangiferin exerts its antidiabetic effects by reducing insulin resistance [42].
Figure 7: Chemical structures of mangiferin [42].
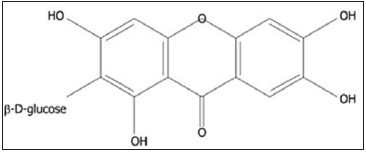
Figure 8: Building blocks of procyanidins [43].

Procyanidins: procyanidins are polymers or oligomers of the proanthocyanidin (or condensed tannins) class including the catechins, epicatechins, gallocatechin, and epigallocatechin. The antidiabetic effect of both A- and B-type procyanidin oligomers in different Cinnamon species has been demonstrated in high-fat diet-fed and low-dosest reptozotocin-induced diabetic mice when administered for 14 days (Figure 9). Furthermore, procyanidins increased the consumption of extracellular glucose in insulinresistant HepG2 cells and normal HepG2 cells [43].
Figure 9: Chemical structure of phytoestrogens [44].
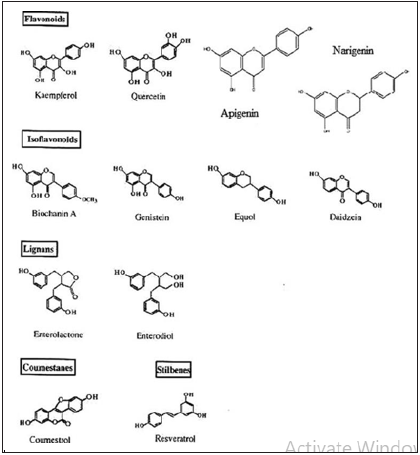
Phytoestrogens: based on their chemical structure, are a heterogeneous group of polyphenolic plant compounds that can be classified into four main groups including isoflavonoids, flavonoids, stilbenes, and lignans. Recently, the effect of phytoestrogen, (3R)- 1, 7-diphenyl-(4E, 6E)-4,6-heptadien-3-ol isolated from Curcuma comosa Roxb (Zingiberaceae) on insulin resistance has been investigated in prolonged estrogen-deprived rats (Figure 10). The findings demonstrated that treatment for 12 weeks with this compound markedly reduced serum total cholesterol and lowdensity lipoprotein levels, improved insulin sensitivity and partially restored uterine weights in rats [44,45].
Figure 10: Chemical structure of Vescalagin [46].
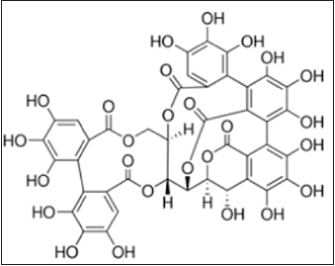
Vescalagin, also known as vescalene or castalagin, belongs to the class of organic compounds known as hydrolyzable tannins. Vescalagin, isolated from Psidium guajava L. (Myrtaceae), alleviated the insulin resistance in insulin-resistant mouse hepatocytes [46].
Ginsenosides or panaxosides are a class of natural product steroid glycosides and triterpene saponins (Figure 11). Ginsenosides from Panax ginseng C.A. Mey (Araliaceae) are the main biological active components for the antidiabetic effect [47]. Its metabolic activity is not well understood. However, it is believed that the mode of action includes enhancement of insulin sensitivity due to lesser insulin demand. Besides, Panax ginseng will stimulate insulin signaling pathway such as protein kinase B and insulin receptor substrate-1 in order to increase secretion from pancreatic β-cells. Its hyperglycemic effect also includes in enhancing gastrointestinal absorption by intestinal bacteria. Increase of translocation of Glucose Transporter Type 4 (GLUT 4) to cell membrane will also enhance the glucose absorption as well as glucose utilization [31].
Figure 11: Chemical structure of Vescalagin [46].

Oligosaccharides: Oligosaccharides are a class of carbohydrates possessing 2–10 monosaccharide units. The rhizome of Zingiber officinale Rosc (Zingiberaceae) rich in oligosaccharides as bioactive components can enhance glucose uptake in cultured L6 myotubes (Figure 12). The investigation suggested that the phenolic gingerol and polysaccharides were the major active constituents enhancing glucose absorption [48,49]. The phytoconstituents and their antidiabetic activities were compared to those of plants used against diabetic in Cameroon. From these comparison nineteen plants among plants used by patients of gestational diabetes were confirmed antidiabetics by the presence of antidiabetic known constituents in their chemical constitution or their previous effects antidiabetic.
Figure 12: Oligosaccharides with 2-10 monosaccharide units [49].

Usual antidiabetic plants used in Cameroon and their ethnopharmacological preparation
Many of these plants may possess antidiabetic substances belonging to the described above phytochemical groups enhancing insulin sensitivity, insulin resistance in comparison of antidiabetic plants from literature. There are:
Aloe spp rich in aloin and aloe emodin improving insulin sensitivity [31]. Drink a half glass of Aloe spp gel completed with water two times daily.
Adansonnia digitata L. (Bombacaceae) rich in Glycosides, tannins, alkaloids, lupeol, semigossypal may be increasing insulin sensibility [31,48]. In Cameroon, stem bark or leaves are prepared in decoction. Drink two glasses daily.
Allium cepa L. (Liliaceae) contains several antidiabetic compounds including Allyl propyldisulfide S-methyl cysteine sulfoxide and pentosides-barbaloin, isobarbaloin, aloin, betabarbaloin which present the following antidiabetic activities: Hypoglycemic, hypocholesterolemic and hyperlipidemic effect; the plant extract has proved to medicate diabetic nephropathy by lowering blood cholesterol levels and decreasing lipid peroxidation [50,51]. Allyl propyl disulphide known as APDS inhibits the insulin destruction by the liver and provokes the production of insulin by the pancreas [52]. Due to the presence of pentosides-barbaloin, isobarbaloin, aloin, betabarbaloin Allium cepa may control insulin sensitivity [52]. In Cameroon, 50g of pounded oinion are prepared in infusion. Drink two glasses daily.
Allium sativum L. (Liliaceae) contains the following constituents llicin (diallyldisulfide-oxide) and S-allyl cysteine sulfoxide which prove many antidiabetic activities including prevent diabetic cardiovascular complications; stimulate the secretion of insulin from pancreatic B cells, sparing insulin effect, increasing glucose utilization, hydroxy methyl glutaryl CoA reductase inhibitor, antioxidant, anti-inflammatory; Enhancement of insulin sensitivity; stimulate insulin signaling and increase translocation of GLUT4 [52]. In Cameroon, 50g of crushed galic prepared in a glass of hot water (infusion), then drink two glasses daily [52].
Aloe vera (L.) Burm.f. (Xanthorrhoeaceae) possess as phytochemicals Aloin, aloe-emodin, pseudoprototinosaponin AIII, prototinosaponin AIII. Increase secretion of insulin from pancreatic beta cells, antioxidant, anti-inflammatory, inhibiting pancreatic α-amylase activity, increase insulin sensitivity. The main phytochemicals groups that can improve insulin sensitivity are flavonoids, terpenoids, glycosides and oligosaccharides. A polyphenol‐rich Aloe vera extract containing both aloin and aloeemodin was able to significantly decrease body weight and blood glucose levels and protected animals against unfavorable results on insulin resistance when administered orally for a period of 4 weeks to insulin‐resistant mice [31]. In a clinical study, an Aloe vera gel complex has been demonstrated to reduce the fasting blood glucose, body weight and insulin resistance in obese individuals with prediabetes or early diabetes mellitus [37]. Aloin and aloeemodin seem to be involved in this antidiabetic effect [31,53]. In Cameroon water maceration of A. vera leaves is drunk at the rate of two glasses daily
In participants of Artemisia spp group fasting blood sugar, serum insulin levels, insulin resistance assessment homeostasis model and β cell function were significantly reduced. In addition, circulating adiponectin levels were also significantly upregulated, which also contributed positively to the improvement in insulin sensitivity. Daily administration of Artemisia extract improves insulin sensitivity by upregulating adiponectin in women with gestational diabetes mellitus. Reduces insulin resistance as measured by the homeostasis model assessment but the cellular mechanism is not determined. Elevates liver glucokinase, liver glycogen, and reduces liver fat accumulation [54]. In Cameroon water infusion of Aole vera leaves is drunk at the rate of two glasses daily.
Azadirachta indica L. (Meliaceae) Worldwide large numbers of patients are treated by natural neem tablets. Its extract improves the blood circulation by enlarging the blood vessels and useful in reducing the blood glucose level in the body [55]. In Cameroon water decoction of Azadirachta indica bark is drunk at the rate of two glasses daily.
Catharanthus roseus L. (Apocynaceae) contains several phytochemicals including Catharanthaine, vincristine, vinblastine, vindoline and etrahydroalstonine. Oral administration of 500mg/ kg dose of leaves and twigs extract was beneficial in animals for lowering in blood sugar level [56]. The mechanism of action of Catharanthus roseus is increases the synthesis of insulin from β cells of Langerhans [56]. Enhancing intestinal glucose uptake through activity of glucose-6-phosphate dehydrogenase in pentose phosphate pathway [57]. Improvement of activity of glucokinase which facilitates Phosphorylation of glucose to glucose-6- phosphate to possess glycemic control [56]. Better utilization of glucose by liver. Antioxidant is capable to prevent important organ damage by free oxygen radicals [57]. Vindoline in Catharanthus roseus is known as protein tyrosine phosphatase-1B inhibitor which mimics the action of insulin as well as increases the insulin sensitivity by elevating the phosphorylation of insulin receptor. Besides, its direct antidiabetic effect. It is strongly associated with inhibition of α-glucosidase in the gastrointestinal environment that subsequently inhibit hydrolyses of carbohydrates [31]. In Cameroon water decoction of Catharanthus roseus L leaves is drunk at the rate of two glasses daily.
Cinnamomum zeylanicum Blume (Lauraceae) contains the following substances Tannins, flavonoids, glycosides, terpenoids, coumarins and anthraquinones. This plant affects the absorption of carbohydrates from the gastrointestinal environment by inhibiting α-glucosidase and α-amylase [57]. In Cameroon water decoction of Cinnamomum zeylanicum leaves is drunk at the rate of two glasses daily.
Canna indica L: (Cannaceae) stimulates the glucose transport by stimulation of GLUT1 protein synthesis and the activation of PI3K. In Cameroon water decoction of Canna indica leaves is drunk at the rate of two glasses daily.
From Corchorus olitorius L. (Tiliaceae) Caffeic acid, chlorogenic acid and isorhamnetin were isolated. This plant showed the inhibition of α-amylase, α-glucosidase & ACE (IC50=17.5μgmL-1, 11.4μgmL-1 & 15.7μgmL-1, respectively) [56]. In Cameroon the consummation of Corchorus olitorius leaves in a soup is taken voluntary a day.
Curcuma longa L. (Zingiberaceae) possess α-phellantrene and tripinolene as major phytochemicals. Curcuma longa lowers blood sugar, increases glucose metabolism and potentiates insulin activity and significantly reduces serum insulin level together with increased insulin sensitivity. Powdered form is taken in the rate of 5g tree times daily [57].
Holarrhena antidysenterica (linn.) Wall (Apocynaceae) contains Gallic acid and quercetin as active ingredients. In vitro this plant inhibits α-glucosidase (IC50=0.52mgml-1) and in vivo it decreased postprandial hyperglycemia [57]. In Cameroon water decoction of Holarrhena antidysenterica leaves is drunk at the rate of two glasses daily.
Mangifera indica L. (Anacardiaceae) mangiferin, β-carotene and α-carotene were isolated from Mangifera indica. This fruit tree reduces the intestinal absorption of glucose and inhibits carbohydrate digesting enzymes, aldose reductase and lipid peroxidation [58]. For numerous authors the ethanolic, methanolic and aqueous extracts of seeds, steam-bark and leaf of Mangifera indica revealed antidiabetic property. Among all phytochemical constituents Mangiferin was found as a major chemical which is responsible for anti-diabetic activity and reduced the serum cholesterol rate in diabetic rats [59]. In Cameroon water decoction of Mangifera indica, leaves are drunk at the rate of two glasses daily. Momordica charantia L. (Cucurbitaceae) possess very important antidiabetic compounds including Momordic I, Momordic II, Cucurbitacin B., Charantin, sterol and Lectin. Lectin is non protein which is linked to insulin receptors. This lectin decreases the blood sugar level by acting on peripheral tissues [55]. The plant presents so many activities including the stimulation of glucose utilization, protection of B cell, downregulation of MAPKs and NF- κB, upregulation of PPAR, modulation of PTP1B, enhance glucose uptake, stimulation of insulin secretion. It was suggested that the novel effect of this extract may reduce not only hepatic glycogenesis but also increase glucose utilization in periphery. It can inhibit α-amylase and α-glucosidase in vitro and reduce blood glucose level in Streptozotocin-induced diabetic rats when they were given orally [31]. Water fruit extract of Momordica charantia (200mg/kg) is took two times daily to control successfully diabetes by patients in Cameroon.
Ocimum basilicum L. (Lamiaceae) contains the following compounds Cardiac glycosides, flavonoids, glycosides, reducing sugars, saponins, steroids and tannins. This aromatic herb inhibits α-amylase rat intestinal maltase and sucrase, porcine pancreatic amylase (IC50=21.31mgml-1, 36.72mgml-1 & 42.50mgml-1 respectively) [60]. The presence of these compounds shows that Ocimum basilicum can increase insulin sensitivity. In Cameroon water decoction of Ocimum basilicum leaves is drunk at the rate of two glasses daily.
Panax ginseng C.A. Mey (Araliaceae) stimulates insulin signaling pathway such as protein kinase B and insulin receptor substrate-1 in order to increase secretion from pancreatic β-cells. It enhances of insulin sensitivity due to lesser insulin demand. It also enhances gastrointestinal absorption by intestinal bacteria. Panax ginseng increases translocation of Glucose Transporter Type 4 (GLUT 4) to cell membrane and also enhances the glucose uptake as well as glucose utilization Panax ginseng regulates the expression of genes involved in PPAR and inhibites hepatic gluconeogenesis through AMPK activation. It enhances phosphorylation of AMPK and GLUT4 [31]. In Cameroon water decoction of Panax ginseng rhizomes is drunk at the rate of two glasses daily.
Psidium guajava L. (Myrtaceae) contains the following phytocompounds Vescalagin, strictinin, isostrictinin, pedunculagin, Triterpenoid, sponins, Flavonoid glycosides such as strictinin, isostrictinin, pedunculagin, and Glycoprotein. Clinical treatment of diabetes by flavonoid glycosides such as strictinin, isostrictinin and pedunculagin improves the sensitivity of insulin and increases glucose uptake [61,62]. Aqueous extract of Psidium guajava leaves is drunk in the rate of one glass tree times daily. Zingiber officinale L. (Zingiberaceae) contains Gingerol, shogaol. It Induces a reduction of liver phosphorylase and glucose‐6‐ phosphatase activities, and a significant increase on serum pyruvate level, liver glycogen content, and pancreatic CAMP levels. More investigations must be carried out to evaluate the mechanism of action of medicinal plants with antidiabetic effect. The toxic effect of these plants should also be elucidated [63].
Assessing the 19 antidiabetic plants for Sirtuin 1 activation
The role of Sirtuin 1
The Sirtuin 1 (Sirt 1) is any of seven proteins family of enzymes that functions as a transcription factor for many different physiological processes. The Sirtuin 1 activators have been used for diabetes treatment and the 19 antidiabetic plants presented above need to be assessed for this anti-aging gene activation. So the insulin resistance and organ suicide are closely connected [64]. Food intake or unhealthy diets is regulated by Sirtuin 1, which is essential to maintain the interaction between glucose homeostasis, immune system and numerous nuclear receptors, transcription factors/signaling factors and miRNA intricate in epigenetics with relevance to human diabetes [65]. In the developing countries bacterial lipopolysaccharides is a critical repressor of Sirtuin 1, which is important for the regulation of various human dysfunctions including:
a. stunted growth,
b. obesity
c. cardiovascular disease with effects on NAFLD,
d. inflammation,
e. cognition,
f. mitochondrial biogenesis,
g. neurogenesis,
h. diabetes/cholesterol metabolism, development of MODS,
i. Nitric Oxide (NO) modification with relevance to core body temperature involved with appetite regulation,
j. delayed caffeine clearance linked to the induction of Type 3 diabetes in global populations
k. glucose homeostasis and hepatic xenobiotic metabolism [64-66].
l. Appetite dysregulation includes neurons related with the destruction of the anti-aging gene Sirtuin 1 and other antiaging genes such as Klotho, p66Shc and FOXO1/FOXO3a that are associated to the programmed cell death as mitochondrial apoptosis and glucose dysregulation, lipid and amyloid beta metabolism.
Anti-aging strategies
Activation of SIRT1 by polyphenols have helpful effects on regulation of CR, oxidative stress, inflammation, adipogenesis, cellular senescence, autophagy, apoptosis, circadian rhythm, autoimmunity, skeletal muscle function, metabolism, mitochondria biogenesis and endothelial dysfunction [67]. Activation of Sirtuin 1 results in the multiple advantageous health outcomes, such as enhancement in insulin sensitivity, diminished adiposity, increased mitochondrial functions, reduced glucose levels, and enhanced physiological functions. Anti-aging strategies that involve nutritional diets let neuropeptides and endocrine hormones to preserve cellular and mitochondrial functions to ease nutrient metabolism in the liver and brain. Prevention of insulin resistance has appeared as the primary prevention program in populations worldwide with enhanced zinc intake and maintenance of nitric oxide homeostasis in cells essential to avoid early modifications several anti-aging genes, neuropeptides and endocrine hormones connected with the regulation of appetite, insulin resistance and cellular apoptosis. Anti-aging therapy comprises low-calorie diets that do not contain LPS, mycotoxins or xenobiotics and these diets maintain Sirtuin 1 activity of the brain and liver with appetite regulation thoroughly associated to zinc homeostasis and nitric oxide related to the autonomic control of the liver by the brain [64- 67].
Recorded antidiabetic plants activating Sirtuin 1
Allium sativum L. (Liliaceae) (Garlic) extracts increased the Sirtuin 1 and Sirtuin 2 gene expressions in the livers and kidneys of diabetic rats. Accordingly, it is possible that Allium sativum with its effects on this pathway of gene expression can have antioxidant and anti-inflammatory effects, therefore decreasing diabetic complications [68]. Due to the nearby correlation between these proteins (Sirtuin and UCP) and mitochondrial oxidative phosphorylation, we hypothesized that Moringa. oleifera may exert a protecting effect against the development of diabetes through regulatory effect of Sirtuin 3 and UCP2 [69]. Ethanol extract of Momordica charantia has anti-obesity and anti-diabetic effects through Sirtuin 1 in the liver tissue. Therefore, the physiologically active ingredient of this herb can be used as an anti-diabetic agent to improve glucose metabolism by Sirtuin 1 [70]. Momordica charantia decreased the level of acetylated β-catenin and enhanced the accumulation of β-catenin in the nucleus by increasing the deacetylase activity of SIRT1 [71]. Activation of AMPK and Sirtuin 1 by curcumin isolated from Cucurma longa has also been well-known to mediate the defending effects of curcumin against ischemia/ reperfusion injury, cardiac fibrosis, diabetes, and lipid metabolism anomalies. These protective effects of Sirtuin 1 activation are partly mediated by the deacetylation of p53 and reduction of apoptosis [72]. Two new dammarane triterpenes, 6α,20(S)-dihydroxydammar- 3,12-dione-24-ene: 6α,20(S),25-trihydroxydammar-3,12-dione- 23-ene and one known triterpene 20(S)-ginsenoside Rg3 [5], from Panax ginseng leaves represented a new class of chemical entities that may be able to be developed as Sirtuin 1 activators for antiaging and treatment of age-associated diseases [73]. Mangifera indica (Mango) fruit or fruit extract performances as a Sirtuin 1 activating agent that is having consequently helpful effects to preserve or improve healthy body composition, glucose and lipid metabolism, energy homeostasis, physical power, muscle mass, cell protection and thereby slowing down the aging process and preventing age related chronic diseases [74]. Only 6 plants over 19 recorded may exert a protective effect against the development of diabetes through regulatory effect of Sirtuin 1, which is the more important anti-aging gene [69].
Conclusion
In the present work, we concentrated on antidiabetic plants and their ability to improve insulin sensitivity and insulin resistance, cellular and metabolic properties of these plants were debated. The study also reveals that many antidiabetic plants used in Cameroon may contain antidiabetic compounds with various mechanisms including improvement of insulin sensitivity. These plants present comprehensive details of antidiabetic plants used in the management of gestational diabetes mellitus. The review also demonstrates that the Cameroonian plants highlighted in this work may have potential insulin sensitivity and insulin resistance. This insulin resistance in gestational diabetes is emphasized by obesity and inactivity and can become a severe disorder with important inferences for pregnancy evolution and lasting morbidity for the mother and offspring. Both hormonal, placental, genetic, and epigenetic contributions and modifications are determined by activity, the diet, the microbiome (a vast army of microbes that protects us against germs, breaks down food to release energy, and produces vitamins), the overweight and finally obesity. It has been revealed that the potential plants targeted insulin action globally via several pathways: inhibition of hepatic glucose production or potentiating the peripheral glucose utilization in the muscles and adipocytes by regulating the activity and expression of key enzymes and glucose transporters. Knowledge about the 19 antidiabetic plants used in Cameroon in the treatment of diabetes via the improvement of insulin resistance and insulin sensitivity in pregnancy is extreme importance in the management of gestational diabetes. It is crucial to elucidate with more detailed awareness the mechanisms behind the insulin resistance developing during pregnancy and the impact on the offspring. These plants may be used to adapt the best possible treatment for diabetic pregnant women, beneficial for both the mother and the future generation. Further are still needed to identify. In addition, many other active agents obtained from these plants need advanced studies to be well characterized and reveal the relationship between these active principles and bioactivity. Therefore investigations must be carried out to evaluate the mechanism of action of the 19 medicinal plants with antidiabetic gestational effect. The toxic effect of these plants should also be elucidated. There are not especial plants for gestational diabetes.
Acknowledgement
The authors extend their thanks to their members of family and a chemist for their useful advises supporting this study.
References
- Catalano PM (2010) Obesity, insulin resistance and pregnancy outcome. Reproduction 140(3): 365-371.
- Ulla Kampmann, Sine Knorr, Jens Fuglsang, Per Ovesen (2019) Determinants of maternal insulin resistance during pregnancy: An updated overview. Journal of Diabetes Research 2019: 5320156.
- Hay WW (2006) Placental-fetal glucose exchange and fetal glucose metabolism. Transactions of the American Clinical and Climatological Association 117: 321-340.
- Glueck CJ, Goldenberg N (2019) Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 92: 108-120.
- Khomami MB, Joham AE, Boyle JA, Piltonen T, Arora C, et al. (2019) The role of maternal obesity in infant outcomes in polycystic ovary syn-drome-A systematic review, meta-analysis, and meta-regression. Obesity Reviews 20(6): 842-858.
- Haluzik M (2005) Adiponectin and its potential in the treatment of obesity, diabetes and insulin resistance. Curr Opin Investig Drugs 6(10): 988-993.
- Choi SH, Hong ES, Lim S (2013) Clinical implications of adipocytokines and newly emerging metabolic factors with relation to insulin resistance and cardiovascular health. Front Endocrinol (Lausanne) 4: 97.
- Martineau LC (2012) Large enhancement of skeletal muscle cell glucose uptake and suppression of hepatocyte glucose-6-phosphatase activity by weak uncouplers of oxidative phosphorylation. Biochim Biophys Acta 1820(2): 133-150.
- Catalano PM (2014) Trying to understand gestational diabetes. Diabetic Medicine 31(3): 273-281.
- Skajaa GØ, Fuglsang J, Kampmann U, Ovesen PG (2018) Parity increases insulin requirements in pregnant women with type 1 diabetes. The Journal of Clinical Endocrinology & Metabolism 103(6): 2302-2308.
- Padmanabhan S, Jiang S, Mclean M, Cheung NW (2016) Effect of pregnancy on insulin requirements differs between type 1 and type 2 diabetes: A cohort study of 222 pregnancies. The Australian & New Zealand Journal of Obstetrics & Gynaecology 56(4): 352-357.
- Patterson AG, Gich I, Amini SB, Catalano PM, de Leiva A, et al. (2010) Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction. Diabetologia 53(3): 446-451.
- Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, et al. (2007) Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 30(2): S112-S119.
- Jarvie E, Haugel de Mouzon S, Nelseon SM, Sattar N, Catalano PM, et al. (2010) Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clinical Science 119(3): 123-129.
- Ota A, Ulrih NP (2017) An overview of herbal products and secondary metabolites used for management of type two diabetes. Front Pharmacol 8: 436.
- Vega M, Mauro M, Williams Z (2019) Direct toxicity of insulin on the human placenta and protection by metformin. Fertility and Sterility 111(3): 489-496.
- Chauhan A, Sharma P, Srivastava P, Kumar N, Duehe R (2010) Plants having potential antidiabetic activity: A review. Der Pharm Lett 2: 369-387.
- Weidner C, de Groot JC, Prasad A, Freiwald A, Quedenau C, et al. (2012) Amorfrutins are potent antidiabetic dietary natural products. Proc Natl Acad Sci U S A 109(19): 7257-7262.
- Zhang R, Zhou J, Li M, Ma H, Qiu J, et al. (2014) Ameliorating effect and potential mechanism of Rehmannia glutinosa oligosaccharides on the impaired glucose metabolism in chronic stress rats fed with high-fat diet. Phytomedicine 21(5): 607-614 .
- McIntyre HD, Chang AM, Callaway LK, Cowley DM, Dyer AR, et al. (2010) Hormonal and metabolic factors associated with variations in insulin sensitivity in human pregnancy. Diabetes Care 33(2): 356-360.
- Patel D, Kumar R, Laloo D, Hemalatha S (2012) Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac J Trop Biomed 2(5): 411-420.
- Salimifar M, Hassanabad ZF, Fatehi M (2013) A review on natural products for controlling type 2 diabetes with an emphasis on their mechanisms of actions. Curr Diabetes Rev 9(5): 402-411.
- Chang CL, Lin Y, Bartolome AP, Chen YC, Chiu SC, et al. (2013) Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med 2013: 378657.
- Eddouks M, Chattopadhyay D, Zeggwagh NA (2012) Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evid Based Complement Alternat Med 2012: 142087.
- Prabhakar PK, Doble M (2011) Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin J Integr Med 17(8): 563-574.
- Liu Q, Chen L, Hu L, Guo Y, Shen X (2010) Small molecules from natural sources, targeting signaling pathways in diabetes. Biochim Biophys Acta 1799(10-12): 854-865.
- Bedekar A, Shah K, Koffas M (2010) Natural products for type II diabetes treatment. Adv Appl Microbiol 71: 21-73.
- Malviya N, Jain S, Malviya S (2010) Antidiabetic potential of medicinal plants. Acta Pol Pharm 67(2): 113-118.
- Abbas S, Raza ST, Ahmed F, Ahmad A, Rizvi S, et al. (2013) Association of genetic polymorphism of PPARgamma-2, ACE, MTHFR, FABP-2 and FTO genes in risk prediction of type 2 diabetes mellitus. J Biomed Sci 20(1): 80.
- Goto T, Takahashi N, Hirai S, Kawada T (2010) Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res 2010: 483958.
- Eddouks M, Bidi A, Bouhali BE, Hajji L, Zeggwagh NA (2014) Antidiabetic plants improving insulin sensitivity. Journal of Pharmacy and Pharmacology 66(9): 1197-1214.
- Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal.
- https://www.sciencedirect.com/topics/chemistry/terpenoid.
- Patel DK, Prasad SK, Kumar R, Hemalatha S (2012) An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed 2(4): 320-330.
- Odeyemi SW, Afolayan AJ (2018) Identification of antidiabetic compounds from polyphenolic-rich fractions of bulbine abyssinica rich leaves. Pharmacognosy Res 10(1): 72-80.
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/glycosides.
- Eid HM, Martineau LC, Saleem A, Muhammad A, Vallerand D, et al. (2010) Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol Nutr Food Res 54(7): 991-1003.
- https://www.sciencedirect.com/topics/chemistry/polyphenol.
- Bierhalz ACK, Lopes SA, Pires ALR, Moraes A (2016) Development of polysaccharide-based membranes incorporating the bioactive compound aloin. International Journal of Polymeric Materials 66(4): 193-202.
- https://www.google.com/search?q=what+is+aloeemodin+chemical+groups&client=firefox-b-
- Miura T, Ichiki H, Iwamoto N, Kato M, Kubo M, et al. (2001) Antidiabetic activity of the rhizoma of Anemarrhena asphodeloides and active components, mangiferin and its glucoside 24(9): 1009-1011.
- Pérez Y, Ferrer EJ, Zamilpa A, Valencia MH, Aguilar FJA, et al. (2007) Effect of a polyphenol-rich extract from aloe vera gel on experimentally induced insulin resistance in mice. Am J Chin Med 35(6): 1037-1046.
- Imran M, Arshad MS, Butt MS, Kwon JH, Arshad MU, et al. (2017) Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids in Health and Disease 16(1): 84.
- https://en.wikipedia.org/wiki/Procyanidin.
- Lu Z, Jia Q, Wang R, Wu X, Wu Y, et al. (2011) Hypoglycemic activities of A- and B-type procyanidin oligomer-rich extracts from different cinnamon barks. Phytomedicine 18(4): 298-302.
- Moutsatsou P (2006) The spectrum of phytoestrogens in nature: Our knowledge is expanding. Hormones 6(3): 173-193.
- Prasannarong M, Saengsirisuwan V, Piyachaturawat P, Suksamrarn A (2012) Improvements of insulin resistance in ovariectomized rats by a novel phytoestrogen from Curcuma comosa BMC Complement Altern Med 12: 28.
- https://pubchem.ncbi.nlm.nih.gov/compound/Vescalagin#section=Structures.
- Akhani SP, Vishwakarma SL, Goyal RK (2004) Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol 56(1): 101-105.
- Chang WC, Shen SC (2013) Effect of water extracts from edible Myrtaceae plants on uptake of 2-(n-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose in TNF-α-treated FL83B mouse hepatocytes. Phytother Res 27(2): 236-243.
- https://www.google.com/search?q=what+is+ginsenosides+chemical+groups&source=lmns&client=firefox-b-
- https://www.google.com/search?q=what+is+oligosaccharides+chemical+groups&client=firefox-b-
- Nolé T, Wilfried Lionel TD (2021) An Introduction to the pathways of action of herbal products and secondary metabolites required for the management of diabetes. Interventions Obes Diabetes 4(5): 405-408.
- Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, et al. (2018) An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J Tradit Complement Med 8(3): 361-376.
- Sheela CG, Augusti TK (1992) Indian Journal of Experimental Biology 30: 523-526.
- Al Zuhair HH, El Sayed IM, Sadek AM (1996) Bulletin of the Faculty of Pharmacy (Cairo University) 34: 101-104.
- Verma S, Gupta M, Popli H, Aggarwal G (2018) Diabetes mellitus treatment using herbal drugs. International Journal of Phytomedicine 10(1): 1-10.
- Tiong SH, Looi CY, Arya A, Wong WF, Hazni H (2015) Vindogentianine, a hypoglycemic alkaloid from Catharanthus roseus (L.) G. Don (Apocynaceae). Fitoterapia 102: 182-188.
- Dabe NE, Kefale AT (2017) Antidiabetic effects of artemisia species: A systematic review. Anc Sci Life 36(4): 175-181.
- Samanta S, Chanda R, Ganguli S, Gopi Reddy A, Banerjee J (2019) Anti-diabetic activity of mango (Mangifera indica): A review. 6(2): 23-26.
- Singh SN, Vats P, Suri S (2001) Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol 76(3): 269-277.
- Thu Win MT (2020) Novel effect of medicinal plants on diabetes mellitus. Journal of Diabetes, Metabolic Disorders & Control 7(2): 73-74.
- Deitersen J, El Kashef DH, Proksch P, Storka B (2019) Anthraquinones and autophagy-Three rings to rule them all? Bioorganic & Medicinal Chemistry 27(20): 115042.
- Martins IJ (2017) Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. J Clin Epigenet 3(3): 24.
- Martins IJ (2017) Nutrition therapy regulates caffeine metabolism with relevance to NAFLD and induction of type 3 diabetes. J Diabetes Metab Disord 4: 019.
- Martins IJ (2016) Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research 5(1): 9-26.
- Chung S, Yao H, Caito S, Hwang J, Arunachalam G, et al. (2010) Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys 501(1): 79-90.
- Arab Z, Ziamajidi N, Abbasalipourkabir R, Mohseni R (2018) Garlic (Allium sativum) increases SIRT1 and SIRT2 gene expressions in the kidney and liver tissues of STZ- and STZ+niacinamide-induced diabetic rats. Journal of Basic and Clinical Physiology and Pharmacology 29(5): 463-467.
- Sosa Gutiérrez JA, Valdéz Solana MA, Forbes Hernández TY, Avitia Domínguez CI, Garcia Vargas GG, et al. (2018). Effects of moringa oleifera leaves extract on high glucose-induced metabolic changes in HepG2 cells. Biology (Basel) 7(3): 37.
- Yoon NA, Park J, Lee J, Jeong JY, Kim HK, et al. (2017) Anti-diabetic effects of ethanol extract from bitter melon in mice fed a high-fat diet. Dev Reprod 21(3): 259-267.
- Ma J, Fan H, Cai H, Hu Z, Zhou X, et al. (2021) Promotion of Momordica Charantia polysaccharides on neural stem cell proliferation by increasing SIRT1 activity after cerebral ischemia/reperfusion in rats. Brain Research Bulletin 170: 254-263.
- Zendedel E, Butler AE, Atkin SL, Sahebkar A (2018) Impact of curcumin on sirtuins: A review. Journal of cellular Biochemistry 119(12): 10291-10300.
- Yang JL, Quy Ha TK, Dhodary B, Kim KH, Park J, et al. (2014) Dammarane triterpenes as potential SIRT1 activators from the leaves of Panax ginseng. Journal of Natural Product 77(7): 1615-1623.
- https://patentimages.storage.googleapis.com/4c/aa/3f/e339aebaf9f79a/EP2953485B1.pdf.
© 2021 Nolé T. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






