- Submissions

Full Text
Intervention in Obesity & Diabetes
Relationships between Systemic Inflammation, Oxidative Stress, Endothelial Dysfunction Molecules and Glycemic Control in Non-Insulin Dependent Diabetes
Mohammed H Saiem Al Dahr*
1Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, Saudi Arabia
*Corresponding author: Mohammed H Saiem Al Dahr, Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
Submission:November 09, 2020;Published: November 25, 2020

ISSN 2593-0263Volume4 Issue4
Abstract
Background: Non-insulin dependent diabetes (NIDDM) usually had high risk for vascular dysfunction.
Objective: The target of this study is to measure the relationships between systemic inflammation, oxidative stress and glycemic control in non-insulin dependent diabetes.
Material and Methods: Ninety obese patients with NIDDM (54 males and 36 females). Their age mean was 49.13±5.25 year, their body mass index (BMI) ranged from 31 to 36Kg/m2, and a control group included Nighty healthy volunteers, who was gender and age matched.
Results: Our study results underscores that NIDDM patients had higher significant values of Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) index, glycosylated hemoglobin(HBA1c), Malondialdehyde (MDA), Superoxide dismutase (SOD), Inter-Cellular Adhesion Molecule (ICAM-1), Vascular Cell Adhesion Molecule (VCAM-1), E-selectin, C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α) and Interleukin-6 (IL-6) in addition to lower significant values of the quantitative insulin-sensitivity check index (QUICKI), Glutathione (GSH) and Glutathione peroxidase (GPX) levels in comparison to controls., in addition serum levels of ICAM-1, VCAM-1, E-selectin MDA ,SOD, CRP, TNF-α and IL-6 showed a direct relationship with HOMA-IR and HBA1c. However, serum levels of GSH and GPX showed an inverse relationship with HOMA-IR and HBA1c. However, serum levels of ICAM-1, VCAM-1, E-selectin MDA, SOD, CRP, TNF-α and IL-6 showed an inverse relationship with QUICKI. However, serum levels of GSH and GPX showed a direct relationship with QUICKI.
Conclusion: There is an association between increased systemic inflammation, oxidative stress, endothelial dysfunction molecules and poor metabolic control in NIDDM.
Keywords: Endothelial dysfunction molecules; Cytokines; Metabolic control; Non-insulin dependent diabetes mellitus; Obesity; Oxidative stress Abbreviations: NIDDM: Non-Insulin Dependent Diabetes Mellitus; BMI: Body Mass Index; QUICKI: Quantitative Insulin-Sensitivity Check Index; SOD: Superoxide Dismutase
Introduction
Diabetes mellitus affects about 6% of population where globally more than 550 million subject will have diabetes by 2030 [1]. However, non-insulin dependent diabetes mellitus (NIDDM) through different mechanisms include lack of metabolic control, oxidative stress and inflammatory cytokines induce dysfunction of different body systems as kidney, heart, blood vessels and eye [2,3]. Diabetic complications are related to abnormal levels of oxidative stress biomarkers induced by poor metabolic control [4-8]. Poor control of blood glucose level may induce abnormal levels of oxidative stress biomarkers [9]. β-cell failure, hyperinsulinemia and hyperlipidemia among NIDDM patients induce endothelial dysfunction and abnormal inflammatory markers [10-14]. Low-grade systemic inflammation is involved in the pathogenesis of NIDDM [15] that abnormal level of inflammatory cytokines may impair glucose control and induce insulin resistance [16-20]. Activation of systemic inflammation adversely affects the insulin action that lead to increased pro-inflammatory cytokines expression [21] that in turn induce more inflammatory response and insulin resistance exacerbation [22]. The objective of this study is to measure the relationships between systemic inflammation, oxidative stress and metabolic control in NIDDM.
Materials and Methods
Subjects
Ninety obese patients with NIDDM, the mean of their age ranged from 40-55 years and their body mass index (BMI) ranged from 30 to 35Kg/m2. Smokers and patients with renal insufficiency, congestive heart failure, pregnancy, respiratory failure and hepatitis were excluded. In addition to nighty healthy non-diabetic subjects participated in this study as a control group who was gender and age matched. Before sharing in this study, informed written consent from was signed by all participants.
Laboratory analysis
Glucose control measurement: Hitachi 912 Chemistry Analyzer will be used to measure serum glucose. However, cobas immunoassay analyzer (Roche Diagnostics) will be used to measure serum insulin. Homeostasis model assessment (HOMA-IR)=[fasting blood glucose (mmol/l)_fasting insulin (mIU/ml)]/22.5 is the formula that will be used to calculate the insulin resistance [2]. While the quantitative insulin-sensitivity check index (QUICKI) assessed by the formula: QUICKI=1/[log(insulin) + log(glucose)] is the formula that was used to calculate insulin sensitivity [23].
Inflammatory cytokines measurements: TNF-α and IL-6 were measured using GE Healthcare Amersham, Biotrak Easy ELISA). While enzymatic-colorimetric method with kits (Roche Diagnostics, Mannheim, Germany) were used to measure C-reactive protein (CRP).
Measurement of oxidative stress status: Method of Buege and Aust is the procedure was used to measure malondialdehyde (MDA) and conjugated dienes (CD) as measures for oxidative stress status [24]. However, method of Beutler and colleagues is the procedure was used to measure anti-oxidant status, glutathione (GSH) [25], method of Nishikimi and colleagues is the procedure used to measure glutathione peroxidase (GPx) and superoxide dismutase (SOD) [26].
Statistical analysis
The mean values of the investigated parameters were detected at the beginning and at the end of the study for both groups and they were compared by student paired “t” test. While the unpaired” test was used to compare between the two groups. Pearson or Spearman rank correlation was used to detect the relationship between the investigated parameters (P<0.05).
Results
Baseline data proved no significant differences in the mean values of age and BMI between both groups. While parameters of metabolic control included serum insulin, fasting blood sugar (FBS) and postprandial blood sugar (PPS) levels were higher among NIDDM patients than control subjects (Table 1). Our study results underscores that NIDDM patients had higher significant values of HOMA-IR, HBA1c, MDA, SOD, ICAM-1, VCAM-1, E-selectin, CRP, TNF-α and IL-6 in addition to lower significant values of QUICKI, GSH and GPX levels in comparison to control subjects. Table 2 shows the relationship between parameters of NIDDM patients and the control subjects. Serum levels of GSH and GPX showed an inverse relationship with HOMA-IR and HBA1c. While, serum levels of ICAM-1, VCAM-1, E-selectin MDA, SOD, CRP, TNF-α and IL-6 showed an inverse relationship with QUICKI. However, serum levels of GSH and GPX showed a direct relationship with QUICKI (Table 3).
Table 1: Baseline criteria of all participants.
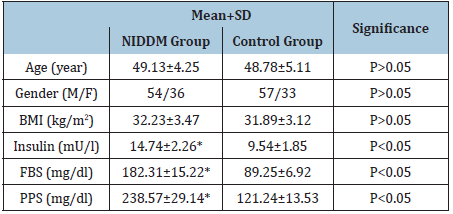
(*)=P<0.05.
Table 2: Mean value and significance of biochemical parameters of NIIDM and control groups.
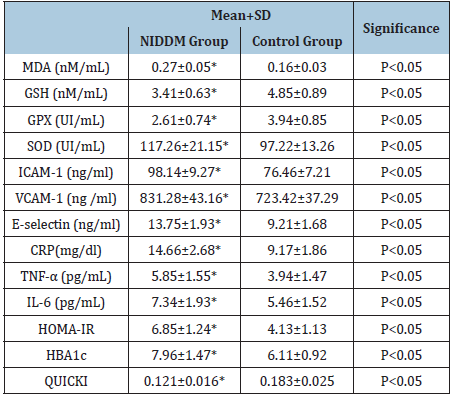
(*)=P<0.05.
Table 3: Relationship between parameters of NIDDM patients and the control subjects.
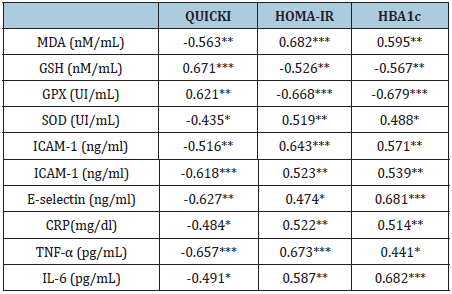
*: P<0.05, **: P<0.01, ***: P<0.001
Discussion
Oxidative stress induces DNA damage in NIDDM [27-30], in addition risk of cardiovascular complications are 2-4 times than non-diabetics [31-33]. Our study underscores that patients with NIDDM had alteration of cytokines, endothelial dysfunction markers and oxidative stress markers, also serum levels of ICAM- 1, VCAM-1, E-selectin MDA, SOD, CRP, TNF-α and IL-6 showed a direct relationship with HOMA-IR and HBA1c. However, serum levels of GSH and GPX showed an inverse relationship with HOMA-IR and HBA1c. Moreover, serum levels of ICAM-1, VCAM- 1, E-selectin MDA, SOD, CRP, TNF-α and IL-6 showed an inverse relationship with QUICKI. However, serum levels of GSH and GPX showed a direct relationship with QUICKI. Several previous studies focused on proving an association between development of insulin resistance and type 2 diabetes [18] and oxidative biomarkers [34,35], inflammatory cytokines [36-38] and endothelial function biomarkers [39-41]. Regarding values of endothelial dysfunction, NIDDM patients had a higher mean values of VCAM-1, ICAM-1 and E-selectin level than control subjects. These findings consistent with Meigs, et al. [39,42] reported that NIDDM patients had endothelial dysfunction [39,42-44]. Moreover, Ferri et al. [45] stated that VCAM-1, ICAM-1, and E-selectin levels were higher among obese subjects than normal subjects [45]. Insulin resistance dyslipidemia were the exact endothelial dysfunction mechanism associated with NIDDM [46].
In the present study, the mean values of MDA and SOD was higher, where the mean values of GSH and GPX was lower among NIDDM patients than normal control subjects. These findings confirmed by Kumawat et al. [47] mentioned that GSH significantly lower and MDA significantly higher among diabetic subjects [47]. Moreover, Kavitha et al. [48] reported that diabetics had higher MDA level [48]. Regarding the inflammatory cytokines, the present study NIDDM patients had significantly higher CRP, TNF-α and IL-6 levels in comparison to normal control subjects. Many studies proved the relation between inflammation and T2DM future development [49,50]. While Liu et al. [51] conducted a meta-analysis included 19 previous studies and proved the link between T2DM and systemic inflammation [51]. Similarly, Julia et al. [52] proved that association between onset of T2DM and endothelial dysfunction & cytokines [52]. Moreover, de Souza Bastos [53] proved that dyslipidemia in T2DM associated with systemic inflammation and oxidative stress [53]. The association between increased systemic inflammation, oxidative stress, endothelial dysfunction molecules and poor metabolic control in NIDDM may be linked to the inactivation of the anti-aging gene Sirtuin 1 that is critical insulin resistance and systemic inflammation. Sirtuin 1 inactivation will lead to loss of metabolic control, increased oxidative stress and inflammatory cytokines dysfunction that lead to multiple organ disease such as diseases of the kidney, liver, heart, brain, blood vessels and eye [54,55]. The possible mechanism that relate insulin resistance to systemic inflammation is complex that may be related to involve increased effect of over produced oxidative stress on the mitochondrial function and endoplasmic reticulum in target tissues [56].
Conclusion
There is an association between increased systemic inflammation, oxidative stress, endothelial dysfunction molecules and poor metabolic control in NIDDM.
References
- Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice 87(1): 4-14.
- (2010) American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 33: S62-S69.
- Al Aubaidy HA, Jelinek HF (2011) Oxidative DNA damage: Antioxidant response in postprandial hyperglycaemia in type 2 diabetes mellitus. Br J Diabetes Vasc Dis 11(2): 87-91.
- Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J (2012) The role of oxidative stress and antioxidants in diabetes complications. Sultan Qaboos Univ J 12(1): 5-18.
- Pan HZ, Zhang L, Guo MY, Sui H, Li H, et al. (2010) The oxidative stress status in diabetes mellitus and diabetes nephropathy. Acta Diabetol; 47: 71-76.
- Odum EP, Ejilemele AA, Wakwe VC (2012) Antioxidant status of type 2 diabetic patients in Port Harcourt, Nigeria. Niger J Clin Pract 15(1): 55-58.
- Shi YC, Pan TM (2012) Red mold, diabetes, and oxidative stress: A review. Appl Microbiol Biotechnol 94(1): 47-55.
- Lima V, Sampaio F, Bezerra D, Neto M, Marreiro N (2011) Parameters of glycemic control and their relationship with zinc concentrations in blood and with superoxide dismutase enzyme activity in type 2 diabetes patients. Arq Bras Endocrinol Metab 55(9): 701-707.
- Likidlilid A, Patchanans N, Peerapatdit T, Sriratanasathavorn C (2010) Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetes patients. J Med Assoc Thai 93(6): 682-693.
- Gómez JM, Vila R, Catalina P, Soler J, Badimón L, et al. (2008) The markers of inflammation and endothelial dysfunction in correlation with glycated haemoglobin are present in type 2 diabetes mellitus patients but not in their relatives. Glycoconj J 25(6): 573-579.
- Ansar S, Koska J, Reaven PD (2011) Postprandial hyperlipidemia, endothelial dysfunction and cardiovascular risk: Focus on in cretins. Cardiovasc Diabetol 10: 61.
- Cersosimo E, DeFronzo RA (2006) Insulin resistance and endothelial dysfunction: The road map to cardiovascular diseases. Diabetes Metab Res Rev 22(6): 423-436.
- Endemann DH, Schiffrin EL (2004) Endothelial dysfunction. J Am Soc Nephrol 15(8): 1983-1992.
- Gómez JM, Sahún M, Vila R, Domènech P, Catalina P, et al. (2007) Elevation of E-selectin concentrations may correlate with potential endothelial dysfunction in individuals with hypopituitarism during therapy with growth hormone. Curr Neurovasc Res 4(1): 55-62.
- Sarangi R, Padhi S, Mohapatra S, Swain S, Padhy RK, et al. (2012) Serum high sensitivity C-reactive protein, nitric oxide metabolites, plasma fibrinogen, and lipid parameters in Indian type 2 diabetic males. Diabetes Metab Synd 6(1): 9-14.
- Calle MC, Fernandez ML (2012) Inflammation and type 2 diabetes. Diabetes Metab 38(3): 183-191.
- King GL (2008) The role of inflammatory cytokines in diabetes and its complications. J Periodontol 79: 1527-1534.
- Mirza S, Hossain M, Mathews C, Martinez P, Pino P, et al. (2012) Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine 57(1): 136-142.
- Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, et al. (2010) Inflammation and the incidence of type 2 diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 33(4): 804-810.
- Vidal PM, Schmid R, Bochud M, Bastardot F, Kanel R, et al. (2012) Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes. The CoLaus study. PLoS ONE 7(12): e51768.
- Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415-445.
- Mezza T, Muscogiuri G, Sorice GP, Prioletta A, Salomone E, et al. (2012) Vitamin D deficiency: A new risk factor for type 2 diabetes. Ann Nutr Metab 61(4): 337-348.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: Insulin resistance and beta cell function from plasma FBS and insulin concentrations in man. Diabetologia 28(7): 412-419.
- Katz A, Nambi SS, Mather K, Baron DA, Follman DA, et al. (2000) Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85(7): 2402-2410.
- Esterbauer H, Gebicki J, Puhl H, Jürgens G (1992) The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med 13(4): 341-390.
- Weckbecker G, Cory JG (1988) Ribonucleotide reductase activity and growth of glutathione-depleted mouse leukemia L1210 cells in vitro. Cancer Lett 40(3): 257-264.
- Masnini M (1988) Determination of superoxide dismutase activity with an electrochemical oxygen probe. Analyt Chim Acta 211: 195-204.
- Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J (2012) The role of oxidative stress and antioxidants in diabetes complications. Sultan Qaboos Univ J 12(1): 5-18.
- Tatsch E, Bochi GV, Piva SJ, De Carvalho JA, Kober H, et al. (2012) Association between DNA strand breakage and oxidative, inflammatory and endothelial biomarkers in type 2 diabetes. Mutat Res 732(1-2): 16-20.
- Mauricio MD, Aldasoro M, Ortega J, Vila JM (2013) Endothelial dysfunction in morbid obesity. Curr Pharm Des 19(32): 5718-5729.
- Seaquist ER (2014) Addressing the burden of diabetes. JAMA: The Journal of the American Medical Association 311(22): 2267-2268.
- Duca L, Sippl R, Snell Bergeon JK (2013) Is the risk and nature of CVD the same in type 1 and type 2 diabetes? Current Diabetes Reports 13: 350-361.
- Lorber D (2014) Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 7: 169-183.
- Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, et al. (2006) Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: Association with dietary intake and plasma concentrations. Am J Clin Nutr 84(2): 395-399.
- Czernichow S, Vergnaud AC, Galan P, Arnaud J, Favier A, et al. (2009) Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Am J Clin Nutr 90(2): 329-335.
- Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, et al. (2007) Genetic variation, creactive protein levels, and incidence of diabetes. Diabetes 56(3): 872-878.
- Krakoff J, Funahashi T, Stehouwer CD, Schalkwijk CG, Tanaka S, et al. (2003) Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care 26(6): 1745-1751.
- Laugsand LE, Asvold BO, Vatten LJ, Romundstad PR, Wiseth R, et al. (2012) Metabolic factors and high-sensitivity creactive protein: The HUNT study. Eur J Prev Cardiol 19(5): 1101-1110.
- Meigs JB, Hu FB, Rifai N, Manson JE (2004) Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 291(16): 1978-1986.
- Czernichow S, Greenfield JR, Galan P, Bastard JP, Charnaux N, et al. (2010) Microvascular dysfunction in healthy insulin-sensitive overweight individuals. J Hypertens 28(2): 325-332.
- Han SH, Quon MJ, Koh KK (2007) Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction. Curr Opin Lipidol 18(1): 58-65.
- Thorand B, Baumert J, Chambless L, Meisinger C, Kolb H, et al. (2006) Elevated markers of endothelial dysfunction predict type 2 diabetes mellitus in middle-aged men and women from the general population. Arterioscler Thromb Vasc Biol 26(2): 398-405.
- Song Y, Manson JE, Tinker L, Rifai N, Cook NR, et al. (2007) Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes 56(7): 1898-1904.
- Laaksonen DE, Niskanen L, Nyyssönen K, Punnonen K, Tuomainen TP, et al. (2004) C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia 47(8): 1403-1410.
- Ferri C, Desideri G, Valenti M, Bellini C, Pasin M, et al. (1999) Early upregulation of endothelial adhesion molecules in obese hypertensive men. Hypertension 34(4): 568-573.
- Shinozaki K, Hirayama A, Nishio Y, Yoshida Y, Ohtani T, et al. (2001) Coronary endothelial dysfunction in the insulin-resistant state is linked to abnormal pteridine metabolism and vascular oxidative stress. J Am Coll Cardiol 38(7): 1821-1828.
- Kumawat M, Pahwa M, Gahlaut V, Singh N (2009) Status of antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus with micro vascular complications. The Open Endocrinology Journal 3: 12-15.
- Kavitha G, Ramani G, Dhass P, Aruna R (2011) Oxidative stress, interleukin (IL-6) and atherogenic index of plasma in diabetic nephropathy. International Journal of Applied Biology and Pharmaceutical Technology 2(2): 211-216.
- Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, et al. (2001) The relation of markers of inflammation to the development of glucose disorders in the elderly: The Cardiovascular Health Study. Diabetes 50(10): 2384-2389.
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286(3): 327-334.
- Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M (2016) Adiponectin, TNF-a and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 86: 100-109.
- Julia C, Czernichow S, Charnaux N, Ahluwalia N, Andreeva V, et al. (2014) Relationships between adipokines, biomarkers of endothelial function and inflammation and risk of type 2 diabetes. Diabetes Res Clin Pract 105(2): 231-238.
- de Souza Bastos A, Graves DT, de Melo Loureiro AP, Júnior CR, Corbi SC, et al. (2016) Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. J Diabetes Complications 30(8): 1593-1599.
- Martins I (2016) Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research 5(1): 9-26.
- Martins I (2017) Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. J Clin Epigenet 3(3): 24.
- de Ferranti S, Mozaffarian D (2008) The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem 54(6): 945-955.
© 2020 Mohammed H Saiem Al Dahr. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






