- Submissions
Full Text
Interventions in Obesity & Diabetes
Framingham Heart Study: A Review of Research Design
Urooj Tahir*
MPHIL (Food & Nutrition Science), University of Punjab, Pakistan
*Corresponding author: Urooj Tahir, MPHIL (Food & Nutrition Science), University of Punjab, Pakistan
Submission: February 07, 2018; Published: February 21, 2018

ISSN: 2578-0263Volume1 Issue1
Contents
Original Cohort
i. Objectives
ii. Sampling
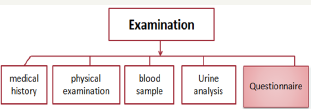
iii. Examination
iv. Maintenance of validity
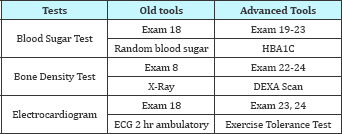
v. Advanced Screening Tools
Comparison of
Original Cohort, Offspring Cohort & New offspring spouse Cohort, Third Generation Cohort, Omni Cohort 1 & Omni Cohort 2
Supporters
i. U.S. Public Health Service
ii. National Heart Institute, NIH
iii. Contract with Boston University and NHLBI
Original Cohort 1948
Cohort study
A group of individuals with a common characteristic or sharing same experiences who are followed over a period of time [1].
e.g. Framingham Heart Study is a prospective investigation of the epidemiology of CHD.
It is a population based open cohort (Figure 1).
Figure 1

Purpose
To study the epidemiology of CHD
Objectives
i. To study the incidence and prevalence and risk factors of cardiovascular disease (CVD)
ii. To study familial patterns , trends in CVD incidence and its risk factors over time
iii. To estimate the incidence rates of disease and to describe the natural history of cardiovascular disease (Figure 2).
Figure 2: Characteristics that made Framingham suitable for a long-term epidemiological study. Feinlieb (1983).

The Framingham investigators have always been aware that the site may not be representative of the United States and have made repeatedly comparisons with other regions to test its generalizability (Figure 3-9).
Figure 3

Sample Size
Total population of town (30-62 years) =10,000.
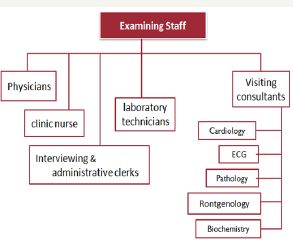
Figure 4: Project Organization.
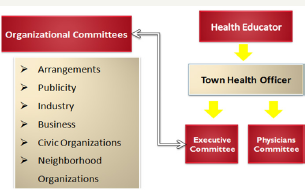
Figure 5
Figure 6
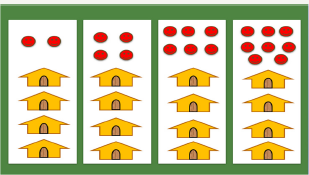
Figure 7
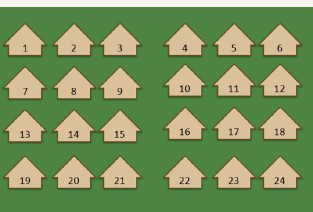
Figure 8
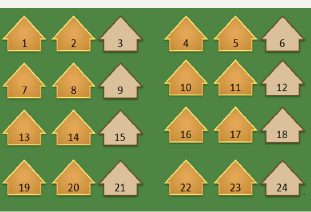
Figure 9: Sampling.
Desired sample size = 6,000
Because only 6,000 exams could be given in this two year cycle
So a 2/3rd sampling ratio was applied to yield the 6,000.
They estimated that 5,000 would be free of CVD at the baseline exam, a large enough sample to produce statistically stable data (Tablel & 2).
Table 1: Response of the Selected Sample.

Table 2: Response Rate 68.7%.

Consent
The documentation of informed consent began in 1971 1st exam cycle of the Offspring Cohort and then the 12th exam cycle of the Original Cohort [2-5]. The research protocols of the Framingham Heart Study are reviewed annually by the Institutional Review Board of the Boston University Medical Center and by the Observational Studies Monitoring Board of the National Heart, Lung and Blood Institute.
I agree to
i. Participate in the FHS clinic examination and studies
ii. Provide a blood sample from which genetic material (DNA and other components) to be used in the genetic studies
iii. To allow researchers from commercial companies to have access to my DNA and genetic data this may be used to develop new lab tests or treatments.
Research Design
Pre-Testing (September 29, 1948)
The FHS examined its own staff members.
Exam (October 11, 1948)
Follow up
Figure 10: Research Design
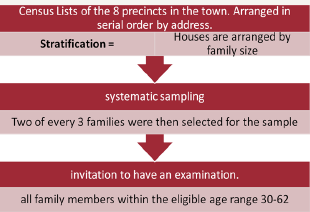
Figure 11: Research Design
Biennial examinations of subjects (for morbidity and mortality development) (Figure 10-17).
Figure 12:
Figure 13:
Figure 14:
Figure 15:

Figure 16:
Validity Maintenance
i. A Clinic Protocol Manual
ii. Periodic "site visits"
iii. A separate Pulmonary Function Manual
iv. Biochemical analysis
Roster of families - first FHS exam records
v. All diagnoses were verified
vi. Review of each death
Figure 17
Failure to appear for re-examination
i. Kept track of the clinical status by community surveillance [6].
ii. Persons who moved out were likely to be healthier
iii. Persons who stopped coming while still resident were seriously, even terminally, ill (Table 3).
Table 3: Advanced Screening Tools.
Offspring Cohort
Figure 18
A prospective epidemiologic study of CHD in young adults to study familial clustering of the disease and its risk factors [7].
1971-1975: Follow up after 4 years (Figure 18 & 19).
Third Generation Cohort
To gather phenotypic and genotypic information
2002-2005: Follow up after every 6 years. Participants in the Offspring Cohort at their 6th and 7th examination cycles provided updated lists:
Figure 19: Recruitment Process.

Family description form
i. Names
ii. Relationships
iii. Residential addresses
iv. Telephone numbers
v. Gender
vi. Birth dates of their children
vii. Identification
viii. Dissemination of study objectives to the community
ix. Invitation
x. Examination of consenting participants
Primary focus
To study large multi generational extended family groups. 3,975 /4,095 participants were members of 879 larger FHS extended families (Figure 20).
Figure 20: Recruitment Process.

New offspring Spouse Cohort
Addition of the data and cell lines of Offspring Spouse Cohort to improve the statistical power for studying families at FHS, 20032005 [8].
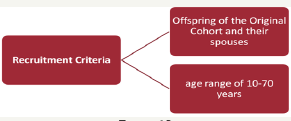
Recruitment Criteria
i. Spouses of Offspring cohort participants who were not initially enrolled
ii. With at least two children in the Third Generation cohort.
Omni Cohort
Omni Cohort 1 1994
Omni Cohort
Omni Cohort
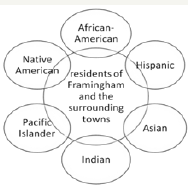
i. Officially known as minority over sampling
ii. Ethnic minority residents of Framingham
iii. A minority group in this study is considered one that is racially distinct from the original cohort, which was defined as white.
Omni Cohort 1
Need to establish a new group of participants reflecting the increasing diversity of the community (Figure 21)
Figure 21: Omni Cohort 1.
Omni Cohort 2 (2005)
Purpose: To allow an ethnically diverse comparable cohort to the Generation III cohort.
Recruitment criteria: No relationship to someone in the first generation had to be claimed to be able to participate (Table 4-8).
Table 4: Purpose

Table 5
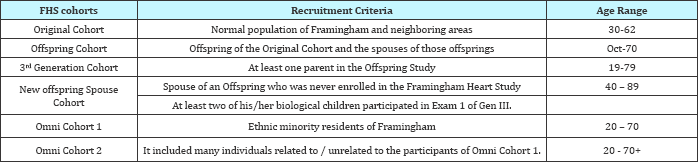
Table 6
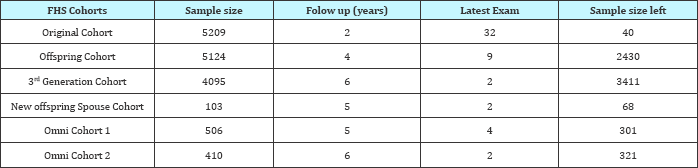
Table 7
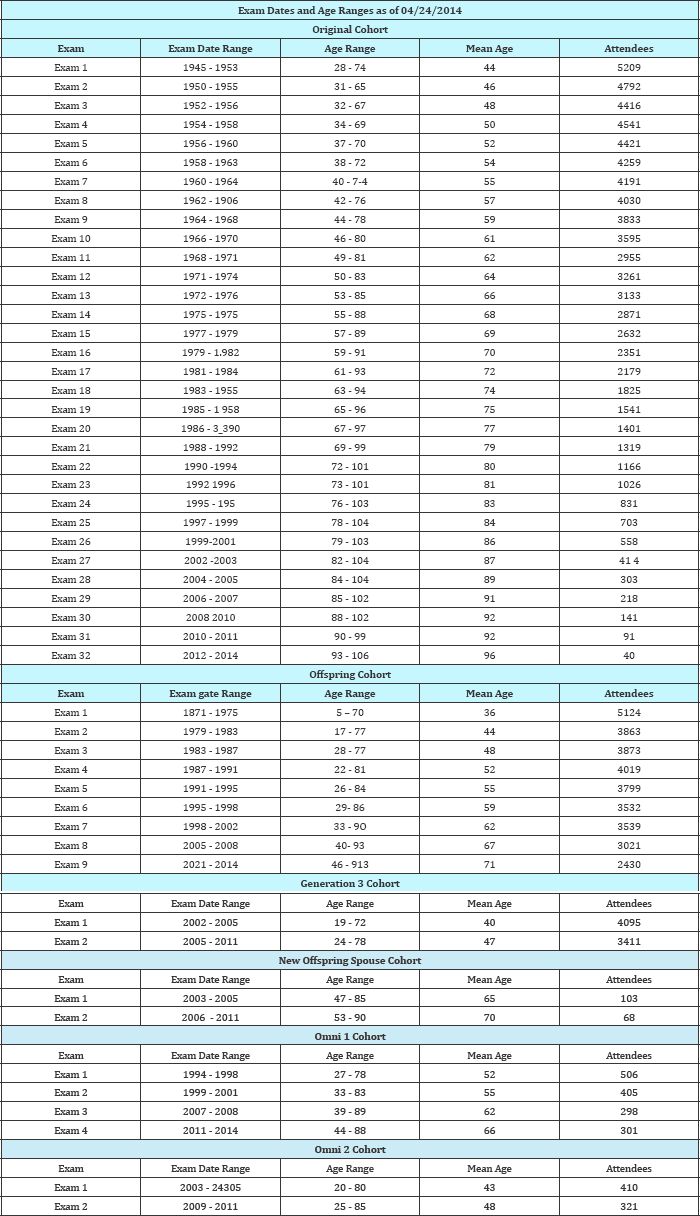
Table 8:
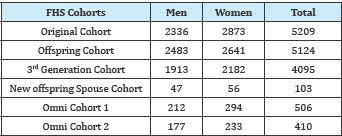
References
- Dawber TR, Meadors GF, Moore FE (1951) Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 41(3): 279-281.
- Framingham Heart Study (2015) A project of the national heart, lung and blood institute and Boston university, USA.
- Mahmood SS, Levy D, Vasan RS, Wang TJ (2014) The framingham heart study and the epidemiology of cardiovascular diseases: a historical perspective. Lancet 383(9921): 999-1008.
- Gordon, Tavia and Kannel, William B (1968) The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease, Section 1 and 2. Bethesda: National Heart Institute.
- Ralph BD & William B. Epidemiological background and design: the framingham study.
- https://biolincc.nhlbi.nih.gov/static/studies/shhs/SHHS%201_M00. pdf#search=omni+cohort+research+design
- Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, et al. (2007). The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: Design, Recruitment, and Initial Examination. Am J Epidemiol 165(11): 1328-1335.
- https://www.framinghamheartstudy.org/researchers/description- data/data/tableofexams.pdf
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






