- Submissions

Full Text
Investigations in Gynecology Research & Womens Health
Clinical Characteristics and Pregnancy Outcome of COVID-19 Infection in Term or Near-Term Singleton Pregnancy: A Retrospective Cohort Study
Cheryl Tam1* and Tsz-Kin LO2
1MBChB, MRCOG, Department of Obstetrics and Gynaecology, Hong Kong
2MBBS, MRCOG, FHKCOG, Cert HKCOG (Maternal and Fetal Med), Department of Obstetrics and Gynaecology, Hong Kong
*Corresponding author:Cheryl Tam, MBChB, MRCOG, Department of Obstetrics and Gynaecology, Princess Margaret Hospital, J304, Hong Kong
Submission:April 04, 2024;Published: April 22, 2024

ISSN: 2577-2015 Volume4 Issue5
Abstract
Objective: Coronavirus disease 2019 (COVID-19) pandemic has affected Hong Kong severely. Local data on COVID-19 infection during pregnancy, particularly peripartum, are limited. This study aims to provide updated data on peripartum COVID-19 infection and investigate its association with mode of delivery and adverse obstetric outcomes.
Methods: This retrospective cohort study included term and near-term pregnant women delivered in a public tertiary hospital in February and March 2022 during the fifth wave of COVID-19 pandemic in Hong Kong. Those with active COVID-19 infection at delivery were compared with those without. Demographic characteristics, vaccination status, and pregnancy outcomes were analysed.
Result: Of the 349 women included in the study, 56 had COVID-19 infection at delivery and 293 did not. Most COVID-19 infections were mild. No significant differences in mode of delivery (p=0.589), postpartum haemorrhage (p=0.171) and ICU admissions (p=1.000) were observed between groups. There was more intrapartum fever in the COVID-19 group (case 26.8% vs control 3.8%, p <0.001). COVID-19 group had longer mean maternal hospital stays (case 3.86 days vs control 3.00 days, p<0.001), mean neonatal hospital stays (case 9.59 days vs control 3.26 days, p <0.001) and increased rate of neonatal fever (case 19.6% vs control 8.2%, p=0.009). Babies’ birth weight, Apgar scores and NICU admission rate were similar between groups (p >0.05). There was no neonatal COVID-19 infection in the maternal COVID-19 group.
Conclusion: COVID-19 infected women appeared to deliver similarly to those without infection. There was no significant difference in mode of delivery with or without COVID-19 infection at delivery.
Keywords: COVID-19; Pregnancy; Term; Peripartum; Caesarean section
Introduction
In early 2020, the World Health Organization (WHO) declared Coronavirus Disease 19 (COVID-19) a worldwide pandemic. Public concern has been raised internationally and global health has been burdened as well. In February 2022, Hong Kong faced a major challenge in dealing with COVID-19 in the fifth wave, driven by the Omicron variant. COVID-19 infections had skyrocketed among pregnant women. Active COVID-19 infection also occurred at delivery, which increased anxiety to pregnant women and clinician about its effect.
Previous studies in the early stage of the COVID-19 pandemic in foreign countries have suggested that COVID-19 infection in pregnancy in general is associated with an increased risk of adverse maternal outcomes, [1,2] including maternal mortality and admission to Intensive Care Units (ICU). Neonates born with maternal COVID-19 infection were more likely to be admitted to the neonatal Intensive Care Unit (NICU) [3]. Higher caesarean section rate in COVID-19 infection has been reported in previous studies in the early COVID-19 era, [1-6] with up to 93% in a report in China [6]. Although there have been many studies investigating the effects of COVID-19 infection on pregnancy, there are relatively few studies that address pregnant women with active infection during delivery [7]. There may be a potential impact of acute infections, or isolated environments, on different aspects of the delivery process, including the mode of delivery.
With the advance of COVID-19 vaccine and emerging COVID-19 virulence, we aim to provide updated data on clinical characteristics of active COVID-19 infection at term or near-term pregnancies to evaluate the effect on mode of delivery and adverse maternal and perinatal outcomes of active COVID-19 infection at delivery.
Methods
Study design
This is a retrospective observational cohort study conducted in the obstetric unit of Princess Margaret Hospital, a public tertiary medical centre in Hong Kong, which is also an Infectious Disease Center of the Hospital Authority. The study was carried out during the largest community outbreak of COVID-19 infection in Hong Kong since the COVID-19 era.
Inclusion and exclusion criteria
All pregnant women delivered in the hospital from 1st February 2022 to 31st March 2022 were screened. All pregnant women delivering a singleton fetus ≥34 weeks of gestation in the period were included. Preterm delivery was excluded as this study focused on term and near-term pregnancies. Multiple pregnancies and COVID-19 infections diagnosed after delivery were excluded from this study. The cohort was divided into two groups, active COVID-19 infections (case) and non-infections (control). Active COVID-19 infection was defined as having a laboratory confirmed positive SARS-CoV-2 PCR test within 14 days before or during delivery. Non- COVID-19 infection control group included both never-infected and recovered pregnant women. Recovered pregnant women had either a recent negative SARS-CoV-2 PCR test or passed at least 14 days after the initial positive SARS-CoV-2 test.
Outcome measures
The primary outcome of this study was the mode of delivery, specifically the caesarean section rate. Secondary outcomes were divided into maternal and neonatal. The maternal secondary outcomes included primary postpartum haemorrhage (i.e. ≥500ml blood loss), venous thromboembolism, ICU admissions, maternal oxygen requirement, and length of hospital stay. The neonatal secondary outcomes included baby birth weight, Apgar scores at 1 and 5 minutes, Neonatal Intensive Care Unit (NICU) admission, neonatal fever, baby length of hospital stay, and positive SARSCoV- 2 test result.
Data collection
Eligible cases were identified from delivery records in the delivery suite. Their computerised and written medical notes were reviewed retrospectively. The corresponding computerised neonatal records were also reviewed. Patient demographics, clinical characteristics, COVID-19 vaccination status and pregnancy and neonatal outcomes were collected and analysed.
Severity of COVID-19 infection was categorized into 4 levels according to signs and reported symptoms, i.e. asymptomatic, mild, moderate and severe. Asymptomatic disease refers to positive SARSCoV- 2 test results but no symptoms. Mild disease was defined as having afebrile upper respiratory tract symptoms, including cough, sore throat, sputum, rhinorrhoea, with or without other somatic symptoms, e.g. Myalgia, headache, vomiting etc. Moderate disease was defined as new onset fever with or without upper respiratory tract symptoms. Severe disease was defined as having shortness of breath or desaturation (i.e. oxygen saturation <94% in room air), with or without fever or upper respiratory tract symptoms.
First trimester ultrasounds were done to confirm the gestational dates of pregnant women. Only the pre-pregnancy BMI was adopted. It would be classified as missing data if the prepregnancy BMI was not available. In our unit, pregnant women with risk factors for gestational diabetes received an Oral Glucose Tolerance Test (OGTT). Blood glucose levels were checked at fasting and two hours after 75g glucose loading. Fasting glucose ≥5.1mmol/L or glucose at 2 hours ≥8.5mmol/L were diagnostic for gestational diabetes. OGTT was repeated at 28 weeks if at-risk women had their first OGTT normal before 24 weeks. There was a universal Group B Streptococcus screening for all pregnant women at 35-37 weeks of gestation. Intrapartum antibiotic prophylaxis would be given to all Group B Streptococcus carriers and preterm deliveries.
During the study period, the hospital adopted a compulsory universal admission screening policy for SARS-CoV-2 PCR test by performing nasopharyngeal swabs and throat swabs for all emergency and elective admissions. SARS-CoV-2 positive pregnant women were admitted to an isolated COVID-designated delivery suite with positive pressure ventilation. They were kept in the same delivery suite prior to, during and after delivery. Dedicated midwives were responsible for providing care in the isolated delivery suite. Cardiotocography (CTG) was monitored centrally in each labour room. Personal protective equipment, including a face shield, N95 respirator mask, gloves, and isolation gown, was required before contact with COVID-19-infected individuals. Mode of delivery for pregnant women was based on obstetric and medical indications. Following birth, newborn babies were evaluated by a paediatrician and admitted to a neonatal ward designated for babies born from mothers with COVID-19 infection. COVID-19- infected mothers were then separated from their newborn babies with no direct contact between the two. Direct breastfeeding was prohibited. After discussing the risks and benefits with the mother, expressed breast milk would be considered with appropriate precautions for expression, transportation and storage. Mothers were encouraged to mobilise and to stay hydrated to reduce the risk of thromboembolism. Mothers at high thromboembolic risk were counselled for prophylactic low molecular weight heparin use during their hospital stay. COVID-19-infected mothers would be discharged to community isolation facilities if they remained stable clinically. If neonatal fever was detected at birth, Pediatricians would follow the same work-up protocol, in which, ear swab and blood would be saved for culture and complete blood count and C-reactive protein would be checked. Penicillin and gentamicin would be given as empirical antibiotics. Antibiotic duration depended on several factors, including the infection risk, the level of C-reactive protein, and the blood culture result. Three sets of SARSCoV- 2 PCR tests for newborns by nasopharyngeal swabs and throat swabs were carried out within 24 hours of life, 24 to 48 hours later and before discharge with at least 24 hours apart from the second set. The baby would be discharged from the hospital when all SARSCoV- 2 PCR tests were negative for COVID-19 infection and could be cared for by a non-infected caregiver.
Ethics approval
This study was approved by the Central Institutional Review Board of the Hospital Authority [Ref. no: KW/EX-22-031 (170- 07)]. The review board waived the requirement of patient consent. Personal data was not used during data retrieval. All the data was in electronic format and encrypted.
Statistical analysis
Data analysis was performed with IBM® SPSS® software version 27. Continuous variables are presented as the mean and Standard Deviation (SD). Categorical variables are presented as numbers (n) and percentages (%). Missing data were excluded from the analysis. Chi-square test, Fisher’s exact test and the independent two-sample T-test were used for analysis. Differences were considered significant when the p-value was <0.05.
Result
A total of 355 singleton pregnancies were delivered during the study period. Six pregnant women were excluded from the study. Three had preterm deliveries before 34 weeks and three had COVID-19 infection diagnosed in the postnatal period. The study included 349 singleton pregnancies, divided into two groups: those with COVID-19 infection at delivery (56/349) and those without (293/349) (Figure 1). The non-COVID-19 infection group consisted of 278 pregnant women who never had COVID-19 infection, and 15 who recovered from COVID-19 infection. The prevalence of COVID-19 infection in this study among pregnant women delivered at term and near-term during the outbreak period was 16.05% (56/349).
Figure 1:Flowchart for case ascertainment.
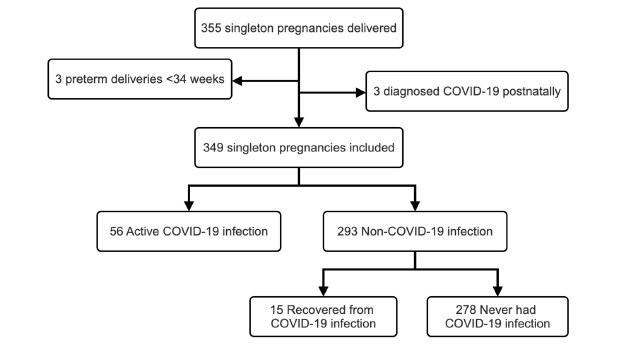
The maternal demographic characteristics, medical comorbidities, obstetric conditions and COVID-19 vaccination status were listed in (Table 1). Missing data were due to incomplete records. There were no significant differences between COVID- 19-infected and non-COVID-infected groups, except for education level (p = 0.048) (Table 1). Majority in both groups received the BioNTech mRNA vaccine (case 63.2% vs control 81.6%, p=0.151) and most of them had received two doses (case 73.7% vs control 67.8%, p=0.557). The mean interval between COVID-19 vaccination and delivery was 8.37 months and 7.11 months in the COVID-19 infection group and non-COVID-19 infection group respectively (p=0.104).
Table 1:Maternal characteristics between COVID-19 group and non-COVID-19 group.
Note: Values are presented as mean (Standard deviation SD) for continuous variables and as n (Percentage %) for
categorical variables
*BMI missing for 2 cases in COVID-19 group and for 1 case in non-COVID-19 group.
†Education level missing for 1 case in COVID-19 and non-COVID-19 group respectively.
‡Smoking status missing for 1 case in non-COVID group.
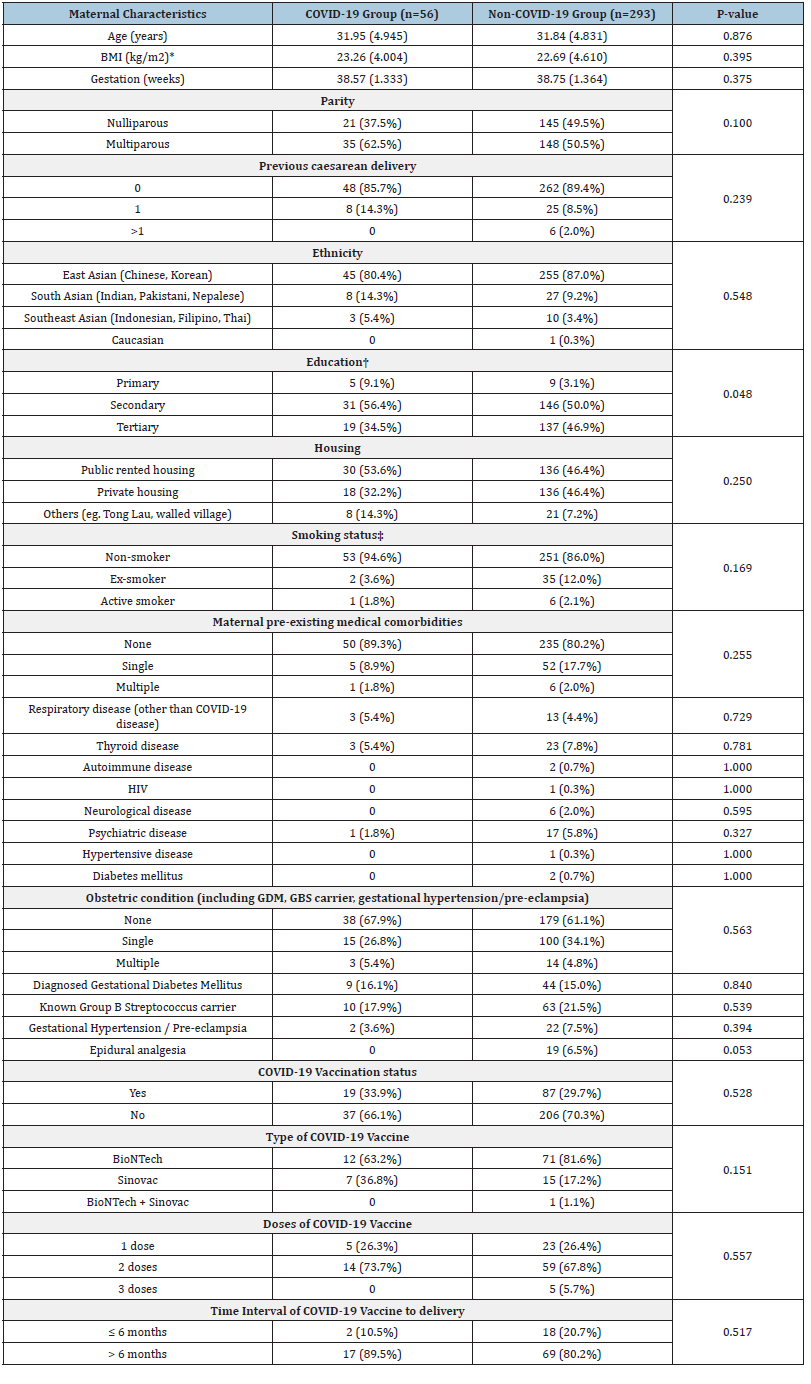
Primary and secondary outcomes
No significant difference in the mode of delivery was observed between COVID-19 infected and non-COVID-19 infected groups (p=0.589) (Table 2). Caesarean section rate, including primary caesarean section, was not increased in the active COVID-19 group. Also, there were no significant differences in the indications for caesarean sections between the two groups (Table 3). The overall rate of labour induction also did not differ significantly between groups (case 39.3% vs control 48.8%, p=0.247). There were no significant differences in the indications for labour induction either (Table 4).
Table 2:Outcomes in COVID-19 vs non-COVID-19 group.
Note: *Apgar score at 1 and 5 minute missing in 4 cases.
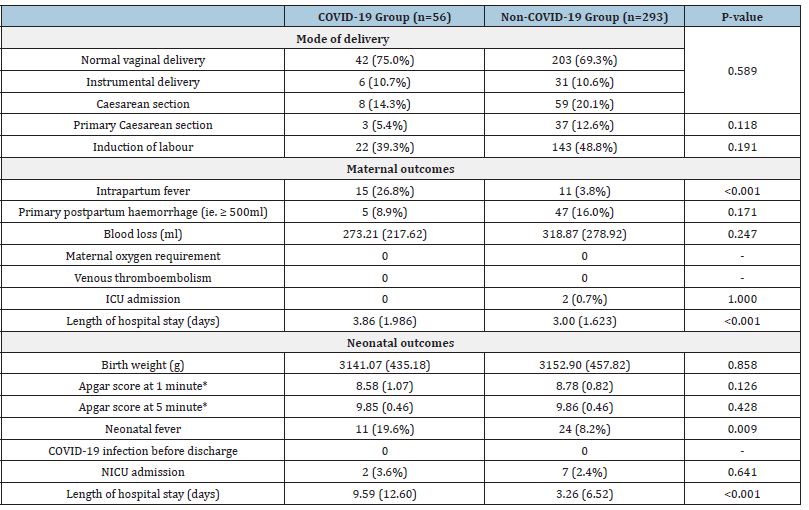
Table 3:Indications of Caesarean section in COVID-19 vs non-COVID-19 group.
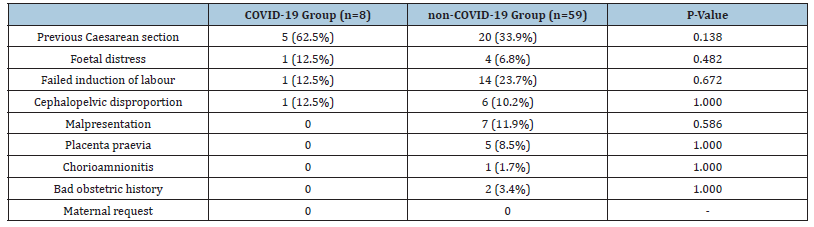
Table 4:Indications of labour induction.
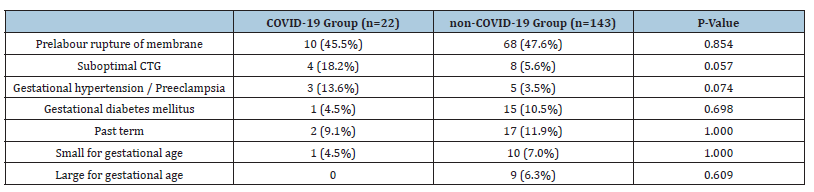
For secondary maternal outcomes, there were no significant differences in the percentages of primary postpartum haemorrhage (case 8.9% vs control 16.0%, p=0.171) and ICU admission (case 0% vs control 0.7%, p=1.000). There were no pregnant women requiring oxygen. None of them had venous thromboembolism. There was a higher rate of intrapartum fever for active COVID-19 infected group (case 26.8% vs control 3.8%, p <0.001), which was likely related to the symptomatology of COVID-19 infection. There was no statistical difference in the epidural analgesia rate between active COVID-19 group and non-infected group (p=0.053).
Active COVID-19 infected women had significantly longer hospital stays (mean 3.86 days for case vs mean 3.00 days for control, p <0.001). The longer maternal hospital stay was also associated with the presence of intrapartum fever (mean 4.31 days with fever vs 3.05 days without, p <0.001).
For secondary neonatal outcomes, there were no significant differences in birth weight, Apgar score at 1 and 5 minutes and NICU admission between the active maternal COVID-19 infection group and non-COVID-19 infection group (Table 2). Four Apgar scores were not available for analysis because the babies were born before arriving at the hospital. Baby’s hospital stay was significantly longer in the active maternal COVID-19 infection group (mean 9.59 days for case vs mean 3.26 days for control, p <0.001). There was a higher neonatal fever rate in the active maternal COVID-19 infection group (19.6% vs 8.2% in the control group, p=0.009). It correlated with a higher maternal intrapartum fever rate among actively infected mothers (p <0.001). However, the longer neonatal hospital stay did not correlate with neonatal fever (mean 4.63 days with fever vs 4.24 days without, p=0.788). No neonates were infected with COVID-19 disease. Septic work-up on all neonates with fever was negative. In the COVID-19 infection group, there were two neonates (3.57%) requiring oxygen support and two (3.57%) requiring CPAP support after delivery. No neonates needed invasive ventilation support in the COVID-19 infection group.
Maternal COVID-19 symptomatology
The common clinical manifestations of COVID-19 infection in pregnant women in this study were cough (n=27, 48.2%), fever (n=19, 33.9%) and sore throat (n=19, 33.9%). Other reported symptoms included sputum (n=9, 16.1%), headache (n=4, 7.1%), vomiting (n=2, 3.6%), myalgia (n=2, 3.6%), and rhinorrhea (n=1, 1.8%). Eighteen (32.1%) infected pregnant women remained asymptomatic. Nineteen (33.9%) infected pregnant women had mild disease and Eighteen (32.1%) infected pregnant women had moderate disease. Only 1 infected pregnant woman had shortness of breath and was classified in the severe group in this study. No infected individuals were reported to have desaturation.
Effect of COVID-19 vaccination
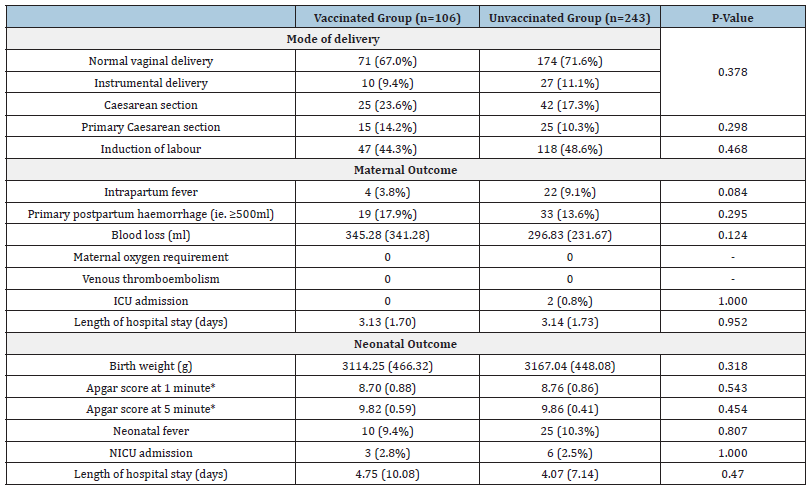
In this study, COVID-19 vaccination uptake was 30.37% (106/349). In the vaccinated group, asymptomatic, mild, moderate and severe diseases were observed in 26.3% (n=5), 42.1% (n=8), 26.3% (n=5) and 5.3% (n=1) respectively. In the unvaccinated group, these were respectively 35.1% (n=13), 29.7% (n=11), 35.1% (n=13) and 0% (n=0). It was found that COVID-19 infection severity did not differ significantly between vaccinated and unvaccinated pregnant women (p=0.433). Between the COVID-19 vaccinated and unvaccinated groups, there were no significant differences in mode of delivery, and adverse maternal and neonatal outcomes (Table 5).
Table 5:Subgroup analysis-Outcomes in COVID-19 Vaccinated vs Unvaccinated group.
Note: *Apgar score at 1 and 5 minute missing in 4 cases.
Discussion
The study found no statistically significant differences in the mode of delivery during active COVID-19 infection at term or near-term. There was no statistically significant increase in adverse delivery outcomes. The finding was consistent with a previous small retrospective study studying active infected and recovered pregnant women [7]. An earlier larger retrospective cohort study found that the increase in caesarean section rate was associated with moderate or severe COVID-19 disease [1]. Our study group had milder COVID-19 disease severity, which may explain why caesarean section rates were not different. On the other hand, previous meta-analysis suggested that the rate of caesarean delivery was not explained solely by the severity of maternal disease or fetal compromise [4]. The indications for caesarean section in our cohort were therefore investigated. One cross-sectional study had suggested that the increased caesarean section during the pandemic was mainly due to maternal requests [5]. In the previous study in China, 61% of the caesarean sections performed for COVID-19 infected pregnant women were caused by concern over the effects of COVID-19 infection on pregnancy [6]. In our unit, the mode of delivery was mainly based on obstetric and clinical conditions, which per se did not seem to impact the mode of delivery significantly.
The length of hospital stay for active-COVID-19 mothers was longer. It may be explained by the presence of maternal intrapartum fever, which required time for investigations and treatment. If fever or severe symptoms persist, a longer hospital stay may be necessary until symptoms subside. In addition, COVID-19 infected individuals had to be transferred to community isolation facilities or home isolation during the pandemic. Additional arrangements were needed to comply with the isolation policy, which likely lengthened the hospital stay. Longer hospital stays for babies from COVID-19 infected mothers was observed. It could be explained by the isolation policy implemented during the COVID-19 pandemic. Newborns of COVID-19 infected pregnant women were transferred to the Pediatric unit for isolation and repeated SARS-CoV-2 tests after birth, prolonging their hospital stays. This neonatal isolation policy was in place because of the unknown risk of transplacental transmission. In this study, there was no vertical transmission of SARS-CoV-2 infection to neonates, which was also consistent with a previous study showing a rare risk of vertical transmission of SARSCoV- 2 to offspring [8]. Therefore, it could be considered to shorten hospital isolation for neonates, or home isolation with uninfected family members. This finding may help policy makers allocate resources more effectively.
The signs and symptoms of COVID-19 infection in term or nearterm pregnancies in this study were also consistent with another study [9], with the most common presentations being cough and fever. This could explain a statistically significant increase in intrapartum fever in the active COVID-19 infection group. It was noted that the majority of infected pregnant women had mild disease. The disease course was less severe than in previous studies published in the early stages of COVID-19 pandemic [1,2]. It could be related to the difference in variant strains of SARS-CoV-2 virus. A previous retrospective multicenter study had suggested that different variants of SARS-CoV-2 virus had different associations with adverse maternal outcomes, with the SARS-CoV-2 Delta variant associated with higher rates of severe maternal comorbidities [10]. During the fifth wave of COVID-19 pandemic in Hong Kong, it was reported that the Omicron variant was more prevalent than other variants [11]. This may explain the milder disease course in this study compared with previous literature.
Some previous studies suggested that CTG changes were observed during maternal COVID-19, including a rise in baseline foetal heart rate, loss of accelerations and decelerations [12,13]. It was postulated to be related to the effects of maternal pyrexia. Despite CTG changes, there was no significant difference in the rate of labour induction for suboptimal CTG between groups in this study. Also, the overall rate of labour induction in both groups did not differ significantly.
Although the government had been advocating COVID-19 vaccination, the uptake in this cohort was lower than expected. It was likely because of limited data on vaccination safety in the early days of the pandemic. In subgroup analysis, both vaccinated and unvaccinated pregnant women showed similar symptomatology and severity of COVID-19 infection. However, our cohort was relatively small and most of our cases were on the mild spectrum of the disease. The effect of COVID-19 vaccination may be underestimated. In addition, a previous systematic review suggested that the COVID-19 vaccine efficacy or effectiveness decreased by 6 months [14]. In our cohort, the mean interval between vaccination and delivery was 7-8 months, which may also account for the similarity in symptomatology and severity between vaccinated and unvaccinated groups. On the other hand, we did not see more maternal or neonatal adverse events in the vaccinated group, which was consistent with the findings in previous systematic reviews [15,16]. This helps address the concern of pregnant women towards COVID-19 vaccination.
Strengths and limitations
This study provided the most updated local data on maternal COVID-19 infection and vaccination, which could help tailor policy making. The data support a cautious return to normalcy. It helps alleviate the disproportional anxiety suffered by the community at large from its severe dread of COVID-19 infection carved by negative reports in the early days of the pandemic, which was predominated by a different variant strain of SARS-CoV-2 virus. In addition, this study focused on peripartum COVID-19 infection in pregnant women, which not many studies have investigated. It should be of interest to most pregnant women as term and nearterm deliveries account for the majority of births.
This study has several limitations. The major limitation is the small sample size. This study was conducted in a single centre, which affected its generalizability as well as its sample size. The statistical power was constrained by the sample size. According to epidemiologic data from the Department of Health in Hong Kong, the COVID-19 infection case number fell rapidly from April 2022 [17]. Our unit also saw the same epidemiologic pattern. Multicenter recruitment can be considered in future research to increase sample size.
The other limitation would be missing data due to the retrospective study design. Potential COVID-19 cases could be missed if they were infected before or during labour but only showed positive COVID-19 test results after delivery.
Since this study only included near-term to term pregnant women, it could not investigate other potential pregnancy complications happening earlier in pregnancy, e.g. Preterm delivery <34 weeks, early-onset pre-eclampsia, and early-onset fetal growth restriction, which were also reported in previous studies [2,18]. Another focused study on preterm COVID-19 infection would be worth exploring. Although this study showed that active COVID-19 infected pregnant women likely would not suffer from more adverse outcomes in the peripartum period, the potential psychological impact of labour in the pandemic was not assessed. The stress of giving birth in an isolation facility could be overwhelming. Also, the impact of severe maternal COVID-19 infection could not be assessed as the cases in this study were relatively mild.
Conclusion
There were no significant differences in the mode of delivery, and obstetric outcomes for pregnant women who had active COVID-19 infection peripartum during the most recent wave of the pandemic at or near term, compared with uninfected pregnant women. Specifically, there was no difference in the caesarean section rate in the active COVID-19-infected group. The majority of COVID-19- infected pregnant women had a mild to moderate disease course, and none required invasive ventilation or ICU admission. There was no vertical transmission of COVID-19 infection.
Acknowledgement
All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and took responsibility for its accuracy and integrity.
Funding/Support
This research received no funding from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Metz TD, Clifton RG, Hughes BL, Sandoval GJ, Grobman WA, et al. (2022) Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA 327(8): 748-759.
- Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, et al. (2021) Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 Infection: the INTERCOVID multinational cohort study. JAMA Pediatr 175(8): 817-826.
- Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, et al. (2020) Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 370: m3320.
- Toro FD, Gjoka M, Lorenzo GD, Santo DD, Seta FD, et al. (2021) Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. ClinMicrobiol Infect 27(1): 36-46.
- Silva CEBD, Guida JPS, Costa ML. (2023) Increased cesarean section rates during the COVID-19 pandemic: looking for reasons through the robson ten group classification system. Rev Bras Ginecol Obstet 45(7): e371-e376.
- Chen L, Li Q, Zhao Y, Jiang H, Qiao J, et al. (2020) More on clinical characteristics of pregnant women with Covid-19 in Wuhan, China. The New England Journal of Medicine 383(7): 696-697.
- Zlatkin R, Dollinger S, Jacoby C, Shmueli A, Barbash-Hazan S, et al. (2022) Obstetric and perinatal outcomes in parturients with active SARS-CoV-2 infection during labor and delivery: a retrospective cohort study. BMC Pregnancy Childbirth 22(1): 511.
- Allotey J, Kew T, Fernández-GarcÃa S, Chatterjee S, Gaetano A, et al. (2022) SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ 376: e067696.
- Delahoy MJ, Whitaker M, O’Halloran A, Chai SJ, Kirley PD, et al. (2020) Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confrmed COVID-19 - COVID-NET, 13 States, march 1-august 22, 2020. MMWR Morb Mortal Wkly Rep 69(38): 1347-1354.
- Mupanomunda M, Fakih MG, Miller C, Ottenbacher A, Winegar AL, et al. (2022) Comparison of severe maternal morbidities associated with delivery during periods of circulation of specific SARS-CoV-2 variants. JAMA Netw Open 5(8): e2226436.
- The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong. (2022) Assessment of Omicron outbreak in Hong Kong.
- Gracia-Perez-Bonfils A, Martinez-Perez O, Llurba E, Chandraharan E (2020) Fetal heart rate changes on the cardiotocograph trace secondary to maternal COVID-19 infection. Eur J Obstet Gynecol Reprod Biol 252: 286-293.
- Sinaci S, Ocal DF, Tokalioglu EO, Ozturk H, Senel SA, et al. (2021) Cardiotocographic features in COVID-19 infected pregnant women. J Perinat Med 50(1): 46-55.
- Higdon MM, Baidya A, Walter KK, Patel MK, Issa H, et al. (2022) Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect Dis 22(8): 1114-1116.
- Carbone L, Trinchillo MG, Girolamo RD, Raffone A, Saccone G, et al. (2022) COVID-19 vaccine and pregnancy outcomes: A systematic review and meta-analysis. Int J Gynaecol Obstet 159(3): 651-661.
- Kontovazainitis CG, Katsaras GN, Gialamprinou D, Mitsiakos G (2023) Covid-19 vaccination and pregnancy: a systematic review of maternal and neonatal outcomes. J Perinat Med 51(7): 823-839.
- Situation of COVID-19 (23 January 2020 to 29 January 2023), The Centre for Health Protection (CHP) of the Department of Health (DH). (2023).
- Wei SQ, Bilodeau-Bertrand M, Liu S (2021) The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 193(16): E540-E548.
© 2024 Cheryl Tam. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






