- Submissions

Full Text
Investigations in Gynecology Research & Womens Health
Shall HPV mRNA Tests be Used for Cervical Cancer Screening?
Yunqing Ma1*, Richard Jun Ma2, Aiguo Zhang1 and Wei Liu1*
1DiaCarta Inc, USA
2California Northstate University College of Medicine, USA
*Corresponding author:Yunqing Ma and Wei Liu, DiaCarta Inc, CA 94588, USA
Submission:March 27, 2024;Published: April 17, 2024

ISSN: 2577-2015 Volume4 Issue5
Abstract
HPV testing is a necessary tool for cervical cancer screening. It mainly includes DNA-based and mRNA- based tests. Here we briefly summarized the commonly used commercial HPV tests and their technologies. We also pointed the better sensitivity of the HPV mRNA test compared to the HPV DNA test. We hope this can help customers to decide their HPV test selection in addition to the preference of their local healthcare authority.
Introduction
According to CDC, every year, 200,000 people are diagnosed with cervical precancer and 9 out of the 10 cervical cancers are caused by Human Papilloma Virus (HPV) [1]. This makes high-sensitivity HPV testing an important screening tool for cervical cancer. Indeed, HPV testing provides heightened sensitivity and enables the earlier detection of cervical lesions with a higher likelihood of progressing to cancer compared to cytology alone. American cancer society has guidelines to screen for cervical cancer starting at age of 25. For people from age 25 to 65, an HPV primary screening by a method such as those approved by the FDA (see below) is done every five years, or an HPV test combined with the Pap (Papanicolaou) test every five years, or the Pap test every three years [2]. Many HPV test based on different technologies are commercially available. Each method has its advantages and disadvantages. We briefly summarized these HPV tests and hope to provide some guidance for clinician and patients to select the appropriate test.
Current HPV Screening Methods
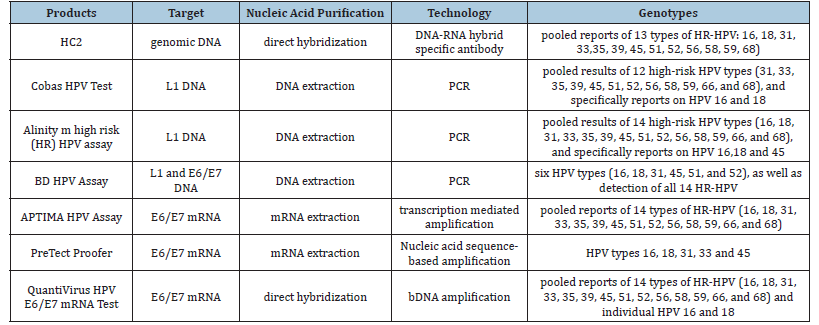
The FDA approved HPV testing methods include DNA-based and mRNA-based assays. The DNA-based methods include Digene Hybrid Capture 2 test (HC2) [3,4], Roche Cobas HPV test [5,6], BD HPV Assay (BD) [7] and Alinity m high risk (HR) HPV [8] assay. The mRNA-based HPV test is the Hologic Aptima test (FDA-approved) [9], PreTect HPV-Proofer assay [10], and QuantiVirus™ HPV mRNA E6/E7 mRNA test.
HC2 test is a direct hybridization-based method. The cervical cells are subjected to an alkaline denaturation solution to release the HPV viral DNA which is hybridized by an RNA probe cocktail for 13 HR-HPV to form DNA-RNA hybrids. These DNA-RNA hybrids are identified through specific antibodies and a chemiluminescent signal is read with a luminometer.
All other HPV DNA testing methods are based on PCR amplification with different primer sets designed in the HPV genome L1 region. This method needs DNA extraction, is suspensible to PCR inhibitors, DNA contamination and subjective data interpretation.
Aptima test is based on Transcription-Mediated Amplification (TMA) technology. This involves the isothermal amplification of E6/E7 mRNA by reverse transcription and subsequent generation of numerous transcripts by RNA polymerase. The test requires mRNA extraction and is performed in a close system. PreTect Proofer is another E6/E7 mRNA detection method which is based on Nucleic Acid Sequence-Based Amplification (NASBA) [10]. NASBA is a two-step process in which specific primers are annealed to RNA template and an enzyme cocktail is used to produce multiple copies of the single stranded RNA.
All the enzyme-based target amplification technology including PCR, TMA and NASBA may give high false-positive results due to template contamination and primer interactions.
QuantiVirusTM HPV mRNA E6/E7 mRNA Test is a CE/IVDcertified, highly sensitive and specific signal amplification nucleic acid probe assay based on bDNA technology [11]. It detects HPV oncogenes E6/E7 mRNA from 14 high-risk type (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) and genotypes HPV 16 and HPV 18 directly from cervical samples (PreservCyt® and SurePath® preservation fluid) for cervical cancer screening [12,13]. This assay is a direct hybridization assay without DNA/RNA extraction or PCR amplification. It significantly reduced the false-positive from the contamination and false amplification that are common in other target amplification methods. It has a higher sensitivity and specificity than Digene method due to bDNA signal amplification and its special oligos design. These methods are summarized in Table1.
Table 1:Commonly used HPV DNA and RNA testing methods.
HPV mRNA testing vs. HPV DNA testing
Although HPV DNA testing has widely been used and only a few mRNA tests are available, it has been demonstrated that the mRNA test is more specific than DNA test in both biology and clinical studies. In biology, HPV mRNA tests using E6 and E7 HPV mRNA is more specific as it detects only the HPV-positive population that is very likely to develop cervical cancer. This is due to the fact that oncogenic potential of HPV infection relies on the production of viral E6/E7 oncoproteins [14]. HPV DNA tests, on the other hand, detect both transient infection and persistent infection of HPV. But at transient infection, the HPV virus is not active, will be cleared, and does not cause cervical cancer. Therefore, there are more false- positive cervical cancer patients that need to be treated and followed up, causing both significant healthcare cost and unnecessary emotional burden to the patients [15,16]. In addition, the follow-up and treatment may lead to fertility problems due to damaged cervix caused by the follow-up required by the HPV false-positive testing results. Clinical studies have also confirmed the better specificity and similar sensitivity of HPV mRNA tests compared to HPV DNA tests [17]. Furthermore, integration of the HPV genome often occurs in the late stages of cervical cancer [18] and L1 region is lost in cancer progression [19]. Thus HPV-DNA method will fail to detect HPV. A recent review of the current mRNA testing supports the hypothesis that HPV mRNA testing is sufficient as the secondary screening tool for cervical cancer [20].
Conclusion
Facing the advantages of HPV mRNA tests, many labs are still insisting on HPV DNA test. Part of the reason is the switch cost of the tests; the other part of the reason is that some labs have ignored the healthcare cost and emotional burden the less specific HPV DNA test has caused. Some of the healthcare providers may even believe following up the false-positive HPV DNA testing patients can reduce the number of people that will develop cervical cancer and would be possibly missed by the HPV mRNA tests. Although there is no clear guideline on which test shall be chosen, many diagnostic testing labs have started or are thinking to switch the HPV tests from DNA testing to mRNA testing. However, the actual benefits of switching still remain to be seen.
References
- Cancers caused by HPV, Human Papillomavirus (HPV), Centers for Disease Control and Prevention.
- The American cancer society guidelines for the prevention and early detection of cervical cancer. American Cancer Society.
- Castle PE, Solomon D, Wheeler CM, Gravitt PE, Wacholder S, et (2008) Human papillomavirus genotype specificity of hybrid capture 2. J Clin Microbiol 46(8): 2595-2604.
- Digene® HC2 High-Risk HPV DNA Test Package
- Cobas® HPV for 4800 System US Package
- Heideman DAM, Hesselink AT, Berkhof J, FV Kemenade, Melchers WJG, et al. (2011) Clinical validation of the cobas® 4800 HPV Test for cervical screening J Clin Microbiol 49(11): 3983-3985.
- BD Onclarity™ HPV Assay US Package Insert [8089894].
- Dhillon SK, Valenčak AO, Xu L, Poljak M, Arbyn M (2021) Clinical and analytical evaluation of the alinity m HR HPV assay within the VALGENTp-3 framework. J Clin Microbiol 59(6): e00286-21.
- Aptima™ HPV Assay US Package
- PreTect Proofer HPV Assay US Package
- Collins ML, Irvine B, Tyner D, Fine E, Zayati C, et (1997) A branched DNA signal amplification assay for quantification of nucleic acid targets below 100 molecules/ml. Nucleic Acids Res 25(15): 2979-2984.
- Shen Y, Gong J, He Y, Cheng G, Okunieff P, et (2013) Quantivirus® HPV E6/E7 RNA 3.0 assay (bDNA) is as sensitive, but less specific than Hybrid Capture 2 test. Journal of Virological Methods 187(2): 288-293.
- Liu J, Yang T, Hu Y, Ye C (2021) The value of HPV E6/E7 mRNA quantitative analysis in distinguishing high-grade cervical squamous intraepithelial lesions from low-grade cervical squamous intraepithelial lesions. J Virol Methods 289: 114014.
- Sørbye SW, Fismen S, Gutteberg TJ, Mortensen ES, Skjeldestad FE (2016) Primary cervical cancer screening with an HPV mRNA test: a prospective cohort study. BMJ Open 6(8): e011981.
- Diaz-Rosario LA (2017) The role of messenger RNA testing in the screening for cervical cancer.
- Yang L, Zhu Y, Bai Y, Zhang X, Ren C (2017) The clinical application of HPV E6/E7 mRNA testing in triaging women with atypical squamous cells of undetermined significance or low-grade squamous intra-epithelial lesion pap smear: A meta-analysis. J Cancer Res Ther 13(4): 613-620.
- Ratnam S, Coutlee F, Fontaine D, Bentley J, Escott N, et (2011) Aptima HPV E6/E7 mRNA test is as sensitive as hybrid capture 2 assay but more specific at detecting cervical precancer and cancer. J Clin Microbiol 49(2): 557-564.
- Wentzensen N, Vinokurova S, Doeberitz MVK (2004) Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res 64(11): 3878-3884.
- Hernandez J, Elahi A, Siegel E, Coppola D, Riggs B, et (2011) HPV L1-capsid protein detection and progression of anal squamous neoplasia. Am J Clin Pathol 135(3): 436-441.
- Macedo ACL, Gonçalves JCN, Bavaresco DV, Grande AJ, Chiaramonte SN, et al. (2019) Accuracy of mRNA HPV tests for triage of precursor lesions and cervical cancer: A systematic review and meta-analysis. J Oncol 2019: 6935030.
© 2024 Yunqing Ma. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






