- Submissions

Full Text
Investigations in Gynecology Research & Womens Health
Update On Cervical Cancer Overscreening in Spain: Review of Current Scientific Evidence on the Topic of Study
Enrique Ricart Torres1*, José Baleriola Júlvez1, Mar Boix Sales2 and Ana Garcia Porcar2
1Specialist in Family and Community Medicine, Spain
2Specialist in Family and Community Nursing, Spain
*Corresponding author:Enrique Ricart Torres, Specialist in Family and Community Medicine, UDMAFYC de Castellón, Spain
Submission:January 22, 2024;Published: January 30, 2024

ISSN: 2577-2015 Volume4 Issue4
Introduction
The following opinion article is part of the authors’ interest in making an approximation to the reality of the current situation of cervical screening in Spain, comparing it with the previous position. The success of the results of the 2017 Spanish National Health Survey [1] (ENSE) on cervical cancer screening coverage prompted the National Health System (SNS) to intensify the early detection strategy in 2019. Based on the opinion of experts and national and international scientific evidence, which is information of great importance, reliable and agreed upon, the following article is written.
To this end, a search for evidence has been carried out in the different reference societies at the Spanish level with regard to cervical cancer screening, such as the Spanish Society of Gynecology and Obstetrics (SEGO) and the Spanish Colposcopy Cervical Pathology Association [2]. (AEPCC) and in databases of interest as well as consulting information from the Spanish National Health System and Primary Care guidelines. As is already known, cervical cancer is the fourth most common neoplasm among women worldwide. Performing screening through cytology has allowed a significant reduction in the incidence and mortality of this cancer.
It has also been confirmed that HPV is the causal agent in practically all neoplasms and precursor lesions, being considered a necessary but not sufficient cause for the development of the disease. The objectives of the early detection strategies for Cervical Cancer (CCU) of the SNS in 2009 were: to optimize the performance of screening tests (cervical cytology every 3-5 years) in medium-low risk women, that is, with a target population of asymptomatic women (25-65 years); and on the other hand, guarantee follow-up through specific programs organized for high-risk women. Considering the results and new evidence in collaboration with scientific societies that deal with this topic, the national screening strategies were updated through the “Cervical Cancer Screening Guide in Spain” in 2019, where the screening coverage throughout the NHS.
Changes in the Coverage of Cervix Cancer Screening
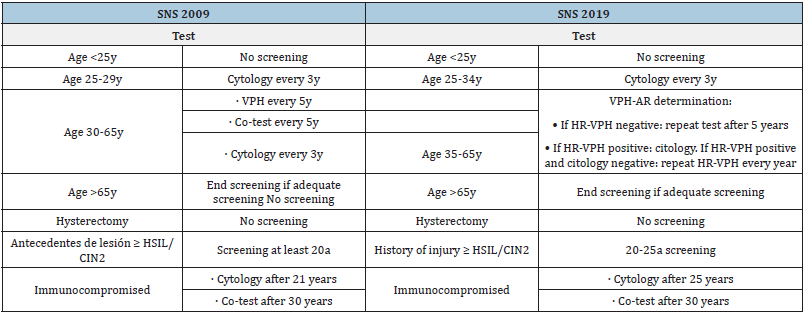
Among the most notable changes in screening See (Table 1), the following stand out: change in age ranges, the inclusion of detection of HR-HPV in addition to subtypes 16 and 18, and performance of co-test only if HR-HPV positive in women between 35-65 years old. Despite commenting that the start of screening is carried out from the age of 25, the guidelines recommend promoting primary prevention by instilling health measures aimed at family planning and STI prevention.
The use of cytology in liquid medium (preferred option by the two guidelines; only with moderate quality of evidence and strong recommendation in favor in AEPCC, allows: reducing the rate of false negatives compared to conventional cytology, making it possible to perform molecular determinations in the same sample (HR-HPV) in a delayed (“reflex”) manner. However, it does not improve sensitivity in detecting intraepithelial lesions. In fact, the use of the HR-HPV test is more effective than cytology in primary screening in women >30 years of age.
Which can extend the screening interval to 5 years in case of negativity. Exhaustive monitoring of ASC-US results does not contribute to reducing costs or CCU. Another notable change in the recommendations is that it opts for population screening, and not opportunistic screening as was being done in some Autonomous Communities. Modification that respects universal and free access to healthcare and does not penalize those people who do not consult (e.g. older people or low-income populations). Finally, it is recommended to start screening in women with HIV infection at age 21 and consider carrying out new check-ups based on their CD4 lymphocytes and/or antiretroviral treatment (Table 1).
Table 1:Comparative table of CCU screening recommendations according to age/comorbidity from the SNS. Own- made table.
Evidence from other Articles
On the other hand, the article “Screening for cervical cancer” [3-5] differs from the other guidelines in terms of the starting age of the target population, as it suggests and recommends the start of screening at 21 years of age.
After a search on Uptodate, different articles of interest regarding CCU screening have been found
A. Giorgi Rossi et al. [6]. comments that the change in
initiation of screening could be assessed in new women who
have been vaccinated against HPV at age twelve; That is to say,
starting at age 30 and using an HPV test could be considered.
If you have been vaccinated at age 15, start screening at age 25
with cytology [6-10].
B. In another article by Petry et al. [11]. conclude that
performing HPV tests for subtypes 16-18 together with
molecular biology techniques (p16/ki-67) can improve the
detection of CCU at a lower cost compared to cytology.
C. In the review of Screening for cervical cancer Penkins
et al. they indicate the age at which screening of the target
population begins at 21 years [12-13].
D. The American Cancer Society (ACS) recommends CCU
screening using the solca HPV test every 5 years from age 25 to
age 65.
Conclusion
After reviewing the changes made in the SNS, it is determined that a strong transformation has been carried out in the interventions carried out in the Spanish territory to improve care as well as to improve the use of resources based on equity and effectiveness of them. Vaccination is a very important primary prevention measure to use and develop throughout the world to prevent the appearance of CCU.
In the future, the implementation of new measures could be considered, still being studied, such as a delay in the start of screening in vaccinated women at the age of 12, before having started sexual relations, or for example being able to develop a technique/intervention in which the patients collected their own sample. It is important to review the following guidelines as scientific evidence advances, to improve the provision of resources within screening programs.
References
- Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, et al. (2010) European guidelines for quality assurance in cervical cancer screening. Second edition-summary document. Ann Oncol 21(3): 448-458.
- Asociación Española de Patología Cervical y Colposcopia (AEPCC). Prevención secundaria del cáncer de cuello del útero, 2022. Conducta clínica ante resultados anormales de las pruebas de cribado.
- Feldman S, Goodman A, Peipert JF (2020) Screening for Cervical Cancer, pp. 1-40.
- García CR, Guillem FC, Seco EM, Gómez Puente JM, José Arango JS, et al. (2022) Lifestyle recommendations. PAPPS 2022 Update. Docusalut 54(1): 102442.
- (2017) Encuesta Nacional de Salud de España (ENSE): Serie de informes monográ
- Rossi PG, Carozzi F, Federici A, Ronco G, Zappa M, et al. (2017) Cervical cancer screening in women vaccinated against human papillomavirus infection: Recommendations from a consensus conference. Prev Med 98: 21-30.
- Guides of practice clinic in cancer gynecological and breast. Sego.
- Luces Lago AM, Mosquera Pan L, López Folgueiras B, Tizón Bouza E (2021) New approach in the screening program for detection early cervical cancer in galicia. Rev Esp Salud Pública 95: 1-11.
- Marzo-Castillejo M, Bartolomé-Moreno C, Bellas-Beceiro B, Melús-Palazón E, Vela-Vallespín C (2022) Cancer prevention recommendations. PAPPS 2022 Update. PAPPS expert groups. cancer prevention recommendations: update 2022. Primary Care 54(Suppl I): 102440.
- Ministerio de sanidad y política social (2010) Estrategia en cáncer del sistema nacional de salud. Sanidad, pp. 114-115.
- Petry KU, Barth C, Wasem J, Neumann A (2017) A model to evaluate the costs and clinical effectiveness of human papilloma virus screening compared with annual papanicolaou cytology in Germany. Eur J Obstet Gynecol Reprod Biol 212: 132-139.
- Cervical cancer screening program. Ministerio de Sanidad.
- Torné Bladé A, Saladrigues MP, Gimferrer CM, Quitllet AF, Ortiz AD, et al. (2014) Cervical cancer screening guide in Spain, 2014. Spanish Journal of Pathology 47: 1-43.
© 2024 Enrique Ricart Torres. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






