- Submissions

Full Text
Investigations in Gynecology Research & Womens Health
Clinical Importance of Pain Sensitization in Gynecology
John Jarrell1, Lina Cadili2 and Lars Arendt-Nielsen3*
1Department of Obstetrics and Gynecology, Canada
2Department of Surgery, Canada
3Center for Neuroplasticity and Pain, SMI, School of Medicine, Aalborg University, Denmark
*Corresponding author: Lars Arendt-Nielsen, Center for Neuroplasticity and Pain, SMI, School of Medicine, Aalborg University, Denmark (LAN@HST.AAU.DK)
Submission: November 18, 2020;Published: January 25, 2021

ISSN: 2577-2015 Volume4 Issue1
Introduction
Peripheral and central sensitizations are conditions of the nervous system that are characterized by increased tone and responsiveness, a lower threshold for the experience of pain and a tendency for pain to persist long after the original stimulus has ended [1]. The mechanism of the development of chronicity is under intense investigation involving a variety of important biochemical and sensitization processes. From a clinical perspective however, the situation can be summed up that pain per se, if not kept under control, may initiate the processes causing the transition from acute to chronic pain.
Basic Investigations
Investigations into the origins of central sensitization in particular, have focused on the role of continuous peripheral drive from the nociceptors (defined as neurons that are activated by noxious stimulation). It has been shown these nociceptors can become sensitized after being injured and develop a lower threshold for activation. Further, the persistence of afferent stimulation and neuronal firing continuously activate the dorsal horn nuclei of the spinal cord and thereby cause central sensitization by e. g. long-term potentiation and neuronal phenotype reorganization. Thus, the central nervous system has the capacity to modify the pain experience such that it can be more or less dependent of the peripheral state, but generally chronic pain patients have some degree of sensitization [2]. There is a disconnection in the relationship of stimulus to response [2] as seen in many chronic conditions where there is a disconnect between pain intensity and size of injure e.g. degree of joint damage in osteoarthritis, size of ulcer, degree of nerve damage and extend of endometriosis [3].
Clinical Investigations
Clinical experience with centralized sensitization has been shown to be present in many clinical conditions such osteoarthritis, rheumatoid arthritis, headache, chronic opioid administration, neuropathic pain and visceral/gynecological pain syndromes [4]. In relation to pelvic pain in women, the known risk factors for non-cyclic pelvic pain are associated with numerous general gynecological and obstetrical factors, including heavy menstrual bleeding, endometriosis, anxiety and depression [5]. Central sensitization can be associated with inflammatory conditions such as endometriosis and interstitial cystitis and can be demonstrated at the bedside by the documentation of allodynia, hyperalgesia and temporal summation of pain from the abdominal wall [6,7].
In a quantitative sensory testing study abdominal allodynia was present in 62.1% women chronic pelvic pain. Allodynia is significantly associated with pain from a visceral origin [8]. Women with allodynia had a significantly greater rate of severe dysmenorrhea and significantly greater duration of severe dysmenorrhea [9]. Temporal summation, present in women with allodynia means that repetitive testing will increase the experience of pain and is a characteristic of central sensitization [10]. Recently a set of clinically applicable bed-side sensory tests was developed for quantitative assessment of hyperalgesia and allodynia the abdominal wall in patients with chronic pain and discovered
the dynamics of such cutaneous stimulation where repeated
stimulation of the hyperalgesic area caused a dramatic increase in
the extent of the allodynic area [11]. The figure below shows the
extension of the allodynic area by dermatome after repeated tactile
stimulations of the hyperalgesic area (Figure 1).
Figure 1:Demonstration of cutaneous allodynia in a woman with severe chronic pelvic pain from embolization of the uterine arteries for post-partum hemorrhage.
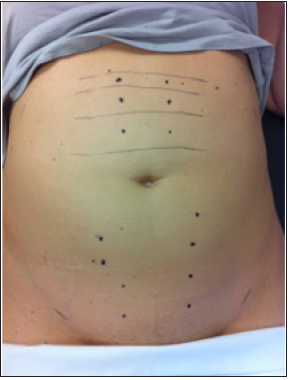
The levels of allodynia are demonstrated to start above the
umbilicus but rise by dermatome with each subsequent pass of the
cotton-tipped applicator. The dots indicate tender areas associated
with the anterior cutaneous nerves as they perforate the abdominal
wall fascia [11]. Hyperalgesia to distention of the uterine cervix
in women with dysmenorrhea has been shown to be associated
with central sensitization [12]. Pain from the female reproductive
organs manifest as referred pain areas according to the so called
Head Zones and the size of those referred pain areas in patients
with chronic visceral/gynecological pain reflect the degree of
central amplification/sensitization of pain [13].
In addition, there modality specific sensory changes are found
in the referred pain area of patients with e.g. dysmenorrhea [14]. A
recent interrogation of this database studied prior pelvic surgery
(caesarian section, hysterectomies, oophorectomies laparoscopies)
in 40 women presenting with chronic pelvic pain [9]. The overall
rate of allodynia was 52%. Those testing negative for allodynia
reported 24 total pelvic procedures in 19 women or 1.31 per
woman while those testing positive for allodynia reported 34
procedures in 11 women or 3.1 procedures per woman. Most of the
difference was due to the frequency of allodynia in laparoscopies (4
of 17 women with allodynia with no previous laparoscopy vs 8 of
16 women with allodynia with at least one previous laparoscopy).
Although this difference may be due to underlying disease
mechanisms, it is possible the procedure itself may contribute to
increased sensitization.
Hyperalgesic Priming
The term hyperalgesic priming designates the process
whereby a prior pain stimulus may evoke the full sensitization
when additional pain is applied [3]. This may be the phenomenon
experienced when women undergo surgery e.g. hysterectomies in
patients with high degree of pre-operative pain and evolve into a
chronic pain state postoperatively [15,16]. Clinical pain conditions
by their nature raise the significant likelihood that repetitive
nociceptive activation reach the central nervous system and
provoke the central sensitization [17]. Experimental pain models
have demonstrated similar changes where peripheral nociceptive
barrage is provoked by the application of algogenic substances such
as capsaicin (chilli) [18]. From an evolutionary perspective, the
recent remarkable secular increase in lifetime exposure to repetitive
menstrual pain may have led to a maladaptive susceptibility to pain
chronicity [19]. Repeated pelvic surgeries and orthopedic revision
surgeries are common and may yet play a role in the development
of increased sensitization [20-22].
A comparative survey explored the frequency of lifetime
surgical intervention among volunteers without chronic pain and
women attending a chronic pain clinic. The study (REB 16-1866
University of Calgary Research Ethics Board) compared a one-time
questionnaire of age, parity, menstrual pain, oral contraceptive
use and lifetime surgical exposure between 21 volunteer women
with no chronic pelvic pain and 21 women attending a chronic pain clinic. The study identified no difference in age, parity or menstrual
pain. Women at the pain clinic had a significantly greater use of
oral contraceptives and a much greater prior surgical exposure.
Volunteers reported two laparoscopies in two women for a total
surgical exposure rate of 2/21 *100=9.5%, those attending the
pain clinic identified 58 procedures among 16 of 21 women=76%.
Among the 16 women who had surgery, there was an average of 3.6
procedures for each woman. Although this is a preliminary study,
it raises the possibility of unintended iatrogenic contribution to
the development of chronic pelvic pain through the processes of
hyperalgesic priming and central sensitization. Further analysis of
this problem is warranted.
Conclusion
The clinical manifestations of central pain facilitation is an important factor for many patients with gynecological disorders such as chronic pelvic pain (e.g. endometriosis, interstitial cystitis, pelvic inflammatory disease), vulvodynia, and dysmenorrhea, in many patients after surgery e.g. hysterectomies, for endometriosis, cesarian section, and gynecological patients undergoing repeated surgeries. The neuroplasticity of the central nervous system and exacerbation of sensitization by repeated surgeries for painful conditions should be critically evaluated.
Acknowledgement
Center for Neuroplasticity and Pain (CNAP) is supported by the Danish National Research Foundation (DNRF121).
References
- Woolf C (2011) Central sensitization: Implications for the diagnosis and treatment of pain. Pain 152(3 Suppl): S2-15.
- Pak DJ, Yong RJ, Kaye AD, Urman RD (2018) Chronification of pain: mechanisms, current understanding, and clinical implications. Curr Pain Headache Rep 22(2): 9.
- Arendt-Nielsen L (2017) Joint pain: More to it than just structural damage? Pain 158 (Suppl 1): S66-S73.
- Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, et al. (2018) Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 22(2): 216-241.
- Latthe P, Mignini L, Gray R, Hills R, Khan K (2006) Factors predisposing women to chronic pelvic pain: systematic review. BMJ 332(7544): 749-755.
- Jarrell J, Malekzadeh L, Yang H, Arendt-Nielsen L (2015) The significance of cutaneous allodynia in a woman with chronic pelvic pain. J Obstet Gynaecol Can 37(7): 628-632.
- Jarrell J, Arendt-Nielsen L (2013) Quantitative sensory testing in gynaecology: improving preoperative and postoperative pain diagnosis. J Obstet Gynaecol Can 35(6): 531-535.
- Jarrell J, Giamberardino MA, Robert M, Esfahani MN (2011) Bedside testing for chronic pelvic pain: discriminating visceral from somatic pain. Pain Res Treat 2011: 692102.
- Jarrell J, Arendt-Nielsen L (2016) Allodynia and dysmenorrhea. J Obstet Gynaecol Can 38(3): 270-274.
- Thompson HD, Tang S, Jarrell JF (2020) Temporal summation in chronic pelvic pain. Obstet Gynaecol Can 42(5): 556-560.
- Jarrell J (2009) Demonstration of cutaneous allodynia in association with chronic pelvic pain. J Vis Exp (28): 1232.
- Arendt-Nielsen L, Madsen H, Jarrell J, Gregersen H, Drewes AM (2014) Pain evoked by distension of the uterine cervix in women with dysmenorrhea: evidence for central sensitization. Acta Obstet Gynecol Scand 93(8): 741-748.
- Arendt-Nielsen L, Laursen RJ, Drewes AM (2000) Referred pain as an indicator for neural plasticity. Prog Brain Res 129: 343-356.
- Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L (2002) A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clin Journal Pain 18(3): 180-190.
- Lunde S, Petersen KK, Andersen ES, Arendt-Nielsen L (2020) Preoperative quantitative sensory testing and robot-assisted laparoscopic hysterectomy for endometrial cancer: can chronic postoperative pain be predicted? Scand J Pain 20(4): 693-705.
- Brandsborg B, Nikolajsen L, Kehlet H, Jensen TS (2008) Chronic pain after hysterectomy. Acta Anaesth Scand 52(3): 327-331.
- Reichling DB, Levine JD (2009) Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 32(12): 611-618.
- Modir JG, Wallace MS (2010) Human experimental pain models 3: heat/capsaicin sensitization and intradermal capsaicin models. Methods Mol Biol 617: 169-174.
- Jarrell J, Arendt-Nielsen L (2016) Evolutionary considerations in the development of chronic pelvic pain. Am J Obstet Gynecol 215(2): 201.
- Jarrell J (2008) Diagnostic and operative laparoscopy in Alberta 1994-2006. J Obstet Gynaecol Can 30(11): 1045-1049.
- Jarrell J (2010) Annual repeat rates of laparoscopic surgery: a marker of practice variation. Am J Med Qual 25(5): 378-383.
- Skou ST, Nielsen TG, Rasmussen S, Simonsen OH, Laursen MB, et al. (2013) Widespread sensitization in patients with chronic pain after revision total knee arthroplasty. Pain 154(9): 1588-1594.
© 2021 Lars Arendt-Nielsen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






