- Submissions

Full Text
Investigations in Gynecology Research & Womens Health
Study of Endometrial Volume and Vascularity by 3D Power Doppler Ultrasound in Women with Perimenopausal Bleeding
Ahmed Sherif1*, Ahmed Ramy M Ramy2 and Rania M Zeid3
Department of obstetrics & gynecology, Ain Shams University, Egypt
*Corresponding author: Ahmed Sherif, Department of obstetrics & gynecology, Ain Shams University, Egypt, Tel: +0122-796-0980; Fax: +2022-256- 9696; Email: ahmedgyna@yahoo.com
Submission: September 02, 2017; Published: October 16, 2017

ISSN: 2577-2015Volume1 Issue1
Abstract
Introduction: Three Dimensional Power Doppler is a useful tool in studying endometrial vascularity. Aim: The purpose of this study is to evaluate the role of 3D - Power Doppler Angiography in the assessment of cases presented with abnormal uterine bleeding in the perimenopausal period and correlates it with histopathological examination of the performed endometrial biopsy to discriminate between benign and malignant endometrial lesions.
Design: Prospective controlled trial.
Methods: 100 patients presented with abnormal uterine bleeding were evaluated by 2 D ultrasound , 3 D power Doppler and had endometrial biopsy.
Results: Based on histopathological examination of endometrial tissue, patients of this study were represented in two main groups (A) Nonmalignant group: (94) and Malignant group: it contained 6 patients with endometrial carcinoma. In the comparisons between non-malignant and malignant group, there was high statistical significant difference (P<0.001) regarding endometrial thickness, endometrial resistive index, endometrial pulsatility index, endometrial volume, endometrial vascularization index, endometrial flow index and endometrial vascularization flow index. In the malignant group, malignancy correlate positively with endometrial thickness (P<0.001), endometrial resistive index (P<0.001), endometrial pulsatility index (P<0.001), endometrial volume (P<0.001), endometrial vascularization index (P<0.001), endometrial flow index (P<0.05) and endometrial vascularization flow index (P<0.001).
Conclusion: The detection of increased endometrial Doppler signals by 3D-PDA may be a possible new ultrasound marker in the diagnosis of endometrial malignancy.
Keywords: Premenopaual bleeding; Abnormal uterine bleeding; Three dimensional power doppler; Endometrial lesion
Introduction
Abnormal uterine bleeding is a common gynecological presentation in outpatient clinic, but is often complex and difficult to diagnose. While most patients have benign diseases, thorough investigation is necessary, particularly in the perimenopausal woman [1].
In perimenopausal women, abnormal uterine bleeding is diagnosed when there is a substantial change in frequency, duration, or amount of bleeding during or between periods [2,3]. There are many benign causes of perimenopausal bleeding, including atrophic endometrium (50%), endometrial hyperplasia (13%) and endometrial polyps (10%) [4]. However, there is also about a 1% probability of cervical cancer and about a 10% probability of endometrial cancer in women with perimenopausal bleeding [5].
Since unrestricted tumor growth is depend upon angiogenesis meaning the process of development of new vessels or the growth Since unrestricted tumor growth is depend upon angiogenesis meaning the process of development of new vessels or the growth of existing ones, thus Doppler ultrasound has been purposed to enhance the ultrasound specificity for endometrial cancer [6]. The value of Doppler and color Doppler U/S in distinguishing benign from malignant endometrial disease is controversial, it has been suggested that low-impedance blood flow at Doppler U/S can be associated with malignancy [7]. Increased focal vascularity may be seen at color Doppler U/S in both benign and malignant diseases of the endometrium [8]. Significant overlap in Doppler indices (i.e. peak systolic velocity, resistive index (RI), pulsatility index (PI)) in benign and malignant endometrial processes reduces the value of Doppler U/S in characterizing endometrial masses [8].
Finally, transvaginal ultrasonography with the “power” angio Doppler is a valuable diagnostic method in cases of early endometrial pathologies. The measurement of blood flow indices in endometrial vessels and uterine arteries is useful to differentiate benign and malignant endometrial pathologies [9]. However, the noninvasive methods for endometrial evaluation are not sensitive enough to exclude endometrial pathology, when invasive methods could not be performed, the combination of transvaginal sonography and power Doppler imaging provided the best results, when both modalities are negative, the probability of cancer is less than 5% [10].
The advent of three-dimensional (3D) power Doppler ultrasound has begun a new era in tissue and organ vascularization research. Using this technique, assessment a virtually reconstructed vascular tree within a volume of interest and can ‘objectively’ determine its vascularization by calculating indices using the specially designed VOCAL TM software [11]. Objective and noninvasive quantification of vascularization of a given tissue volume holds much promise, particularly because this method has proved to be highly reproducible between observers (thereby overcoming one of the main limitations of conventional Doppler ultrasound) [12].
The aim of this work is to measure endometrial volume in women with perimenopausal bleeding through measuring vascularization index (VI), flow index (FI) and vascularizationflow index (VFI) with use of 3D -Power Doppler Angiography and correlate it with histopathological examination of the performed endometrial biopsy to discriminate between benign and malignant endometrial lesions.
Statement of Significance
Problem: Abnormal uterine bleeding in perimenopausal age may be associated with endometrial malignancy
What is already known: Two Dimensional power Doppler has its limitations in predicting endometrial cancer
What this study adds: The use of Three Dimensional power Doppler in patients with perimenopausal bleeding added a new tool for better assessment in vascularity of endometrium
Patients & Methods
This study is a controlled clinical trial that was conducted in Ain Shams University Maternity Hospital, cases were recruited from the outpatient gynecological clinic and 100 perimenopausal women were included in this study. All cases presented by abnormal uterine bleeding. The study was conducted between April 2013 and April 2014.
The study was approved by the hospital ethics committee; approval and informed consent were obtained from the patients before they participated in the study.
The following inclusion criteria were used:
A. Age group (45-55) years.
B. Abnormal uterine bleeding, defined as substantial change in frequency, duration, or amount of bleeding during or between periods.
C. Definitive endometrial histological diagnosis was obtained.
The following exclusion criteria were used:
1. Evident general cause that can cause vaginal bleeding.
2. Presence of vaginal, vulval or cervical causes of bleeding. Each patient was subjected to:
(A) Complete medical history, with assessment of risk factors, such as age, parity, smoking, history of hypertension, diabetes mellitus, as well medication history covering use of HRT, oestrogen, tamoxifen or anticoagulants.
Clinical examination
1. BMI
2. Abdominal examination, looking for abdominal masses and/ or areas of tenderness.
3. Speculum examination to allow assessment of atrophic vaginitis and to rule out tumors of the cervix, vagina or vulva, or cervical polyps.
Transvaginal ultrasound examination
Sonographic examinations were performed in Ultrasound Special Care Unit for the Fetus - Ain Shams University with an expert equipped with Voluson E6 (EXPERT) Ultrasound device which combines the features of wave Doppler Effect technology with pulsed -echo technology and supplied by endovaginal probe, according to a determined scanning protocol. In the lithotomy position with evacuated urinary bladder all examinations were performed with single handed examiner (O.N.M). The probe was inserted into the vagina after complete covering with lubricating gel and protected by a disposable condom. All patients first underwent a standard 2D scan, followed by 3D volume acquisition.
Briefly, first a conventional gray-scale sonography was performed that obtained longitudinal and transverse sections of the uterus and adnexa. Endometrial texture and maximal endometrial thickness (double layer) were measured. After B-mode evaluation is done a 2-Dimensional Power-Doppler gate was activated to assess neo-vascularization from the myometrium and endometrium. Power Doppler settings were set to achieve maximum sensitivity to detect low velocity flow without noise (frequency, 5MHz; power Doppler gain, -7.4; dynamic range, 20-40 dB; edge, 1; persistence, 2; color map, 5; gate, 2; filter, L1; pulse repetition frequency, 0.6kHz). Endometrial Resistive Index (RI) and Pulsatility Index (PI) were measured.
3-Dimensional power doppler examination
Three dimensional volumes were activated to obtain a 3-dimensional box from the uterus. With a sweeping angle of 90 degrees, the acquisition box of 3-dimensional volume was placed over the power Doppler window. The patient was asked to remain as still as possible, and volume acquisition was made during a time interval that varied from 15-20 seconds. Volume acquisition was repeated when artifacts flash-type appeared because of respiratory or intestinal movements.
Volumes were stored and evaluated later in a personal computer. With the virtual organ computer aided analysis (VOCAL) program endometrial area was evaluated manually in the coronal or C plane. With a rotational technique with a 9-degree step, 20 endometrial slices were obtained that outlined the endometrium at the myometrial-endometrial junction from the fundus to the internal cervical os. This process was used because it previously was demonstrated to assay the best reproducibility for endometrial volume and 3-dimensional power Doppler indices. The VOCAL program automatically calculates the endometrial volume and three 3-dimensional power Doppler indices: vascularization index (VI), flow index (FI) and vascularization-flow index (VFI). VI measures the number of color voxels in the volume, which represents the vessels in the tissue and is expressed as a percentage. FI is the mean color value in the color voxels, which indicates the average intensity of blood flow and is expressed as an entire number from 0-100. VFI is the mean color value in all the voxels in the volume, which represents both vascularization and blood flow and is also expressed as an entire number from 0-100.
Endometrial sampling
Within 1 week after ultrasound examination, all patients underwent endometrial sampling by hysteroscopy or office biopsy. Definitive histological diagnosis was obtained in all of the cases that are included in this study. Benign histological findings included cystic atrophy, endometrial polyp, submucous myoma and endometrial hyperplasia. Malignant histologic findings included endometrial cancer. All patients gave verbal informed consent, and all the collected data were statistically analyzed. The association between measured sonographic and Doppler parameters and endometrial pathology were expressed in terms of relative risk and its 95% confidence intervals. Validity of sonographic and Doppler parameters in prediction of endometrial pathology was expressed in terms of sensitivity, specificity, Positive and negative predictive values as well as likelihood ratios. Significance level was set at 0.05.
Sample size
The STATCALC feature of Epi Info™ -Version 6 software was used for calculating the sample size guided by:
Power of the test = 80 %
Confidence level = 95 %
Accepted margin of error = 5 %
Risk percent ratio = 2.5 %
Expected frequency of condition = 10 %
Total sample accepted according to inclusion criteria = 120
Type of the study: Cross sectional study.
Statistical Analysis
Statistical analysis was done on a personal computer using IBM© SPSS© Statistics version 22 (IBM© Corp., Armonk, NY, USA) and MedCalc© version 13 (MedCalc© Software bvba, Ostend, Belgium).
Numerical variables were presented as median and interquartile range and between-group differences were compared using the Mann-Whitney U test. Categorical variables were presented as number and percentage and intergroup differences were compared using Fisher’s exact test. Receiver-operating characteristic (ROC) curve analysis was used to examine the value of 3D-Doppler measures for identification of malignant lesions. The area under the ROC curve (AUC) was interpreted as follows:
AUC = 0.90-1.0: excellent
AUC = 0.80-0.90: good
AUC = 0.70-0.80: fair
AUC = 0.60-0.70: poor
AUC = 0.50-0.60: fail
Comparison of the AUC under various ROC curves was done using the DeLong method.
P < 0.05 is considered statistically significant
Results
Patients in the study were represented in two main groups : (A) Non-malignant group: it contained 94 patients with various endometrial lesions categorized histopathologically to atrophic endometrium 62 patient, endometrial hyperplasia 16 patient [10 patients of them had simple endometrial hyperplasia (10%) while 6 patients had complex endometrial hyperplasia (6%)], endometrial polyps 16 patients [14 patiens of them had simple hyperplastic endometrial polyp (14%), while 2 patients had complex hyper plastic polyp (2%)]. (B) Malignant group: it contained 6 patients with endometrial carcinoma (6%), 2 of them were grade 1(2%) and 4 of them were grade 2 (4%) (Figure 1).
Figure 1: Pie chart showing the percentage of various histopathologic types of lesions.
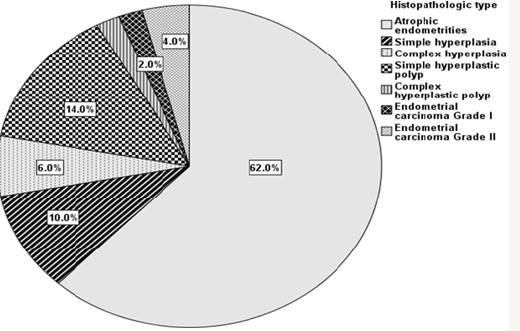
The mean age of the whole study population was 49.3 with a range of 45-55 years. The mean BMI of the whole study population was 30.7The median age of the non-malignant group was (49 years with interquartile range of 47-51 years) which is smaller than the
median of malignant group which was (54 years with interquartile range of 54-55 years), which denoted high statistical significance (P<0.001) .The mean BMI of the whole study population was 30.7. Non-malignant group was having median BMI of 30.3 with interquartile range of (28.7-32.4) which is lower than the median BMI for malignant group whose median BMI was 34.1 with interquartile range of (32.8-34.6), these differences were statistically highly significant (P<0.001).
Discussion
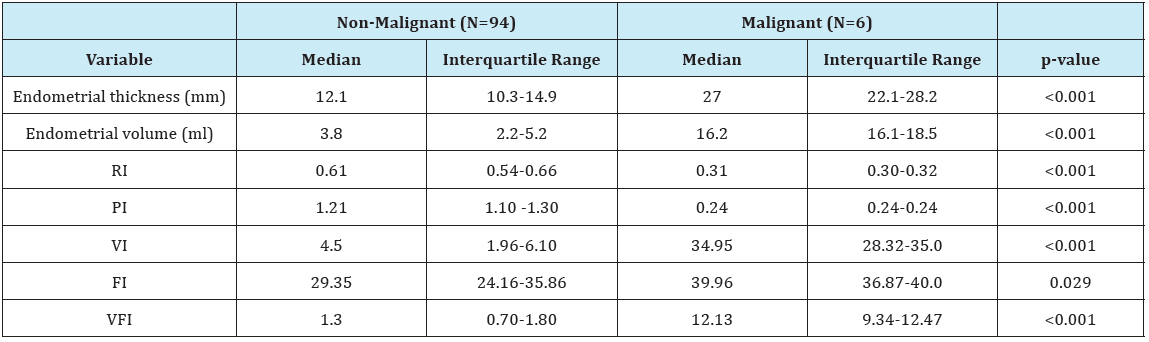
In the current study as regards the endometrial thickness, the median endometrial thickness of whole study population was 12.3 mm with iterquartile range (10.4-17.7) (Table 1), in non-malignant group the median was 12.1 mm with interquartile range (10.3- 14.9), while in malignant group it was much higher 27 mm with interquartile range (22.1-28.2), which denotes high statistical significane (P<0.001) (Table 2). The endometrial thickness cut-off to predict malignancy was >20.02 mm with sensitivity of 100% and specificity of 93.62%. These results are in agreement with the study of Granberg et al. [13] who concluded by measuring the endometrial thickness in 205 women complaining of postmenopausal bleeding that there were no cases of cancer with an endometrial thickness < 9 mm.
Table 1: Endometrial and Doppler measures in the whole study population.
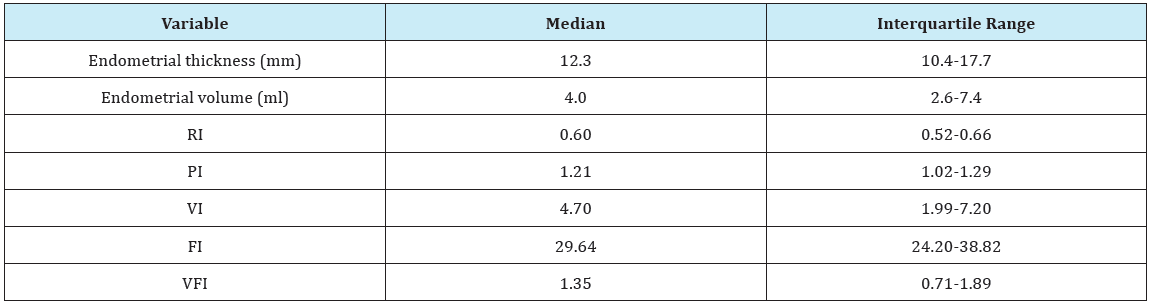
Table 2: Comparison of patients with malignant and non-malignant lesions: 3D-Doppler measures.
We also agree with the study of Osmer et al. 1990 who studied 155 postmenopausal women using 4 mm endometrial thickness cut off limit by vaginal ultrasound have reported a sensitivity of 81% at this endometrial thickness by the vaginal ultrasound in the diagnosis of endometrial neoplasms. Nevertheless, this parameter does not reduce the need for invasive diagnostic techniques because 4% of endometrial cancers would still be missed, with a false-positive rate as high as 50%. In contrast our results aren’t consistent with the study of Saha et al. [14] who found that vaginal ultrasonographic evaluation of endometrial thickness is not sensitive enough to detect cancer in women with postmenopausal bleeding and the study of Tabor et al. [15] who found that using endometrial thickness cut-off <4 mm alone to exclude malignancy isn’t a reliable parameter as 4% of endometrial cancer would still be missed, with false-positive rate as high as 50%.
As regards the results of Doppler velocimetric study of the endometrium in this study, the median resistive index (RI) of the whole study population was 0.6 with interquartile range (0.52- 0.66) (Table 1), in non-malignant group our RI median was 0.61 with interquartile range (0.54-0.66) while in malignant group our median was 0.31 with interquartile range (0.30-0.32), these results show high statistical significane (P<0.001) (Table 1). In our study using RI cut-off ≤0.337 is promising in predicting malignancy with sensitivity, specificity, PPV and NPV of 100% and (P<0.0001) which denotes high statistical significance (Table 2). The results of our study are in agreement with those of Kupesic & Kurjak [16] who reported that low vascular resistance (RI= 0.42±0.02) in the vessels of the central parts of the endometrial lesion as well as the surrounding myometrial vessels if positive for invasion. They suggested that the possible cause is the deficient endothelial membrane and leaky structure. The median pulsatility index (PI) of the whole study population was 1.21 with interquartile range of (1.02-1.29) (Table 3), in non-malignant group the median was 1.21 with interquartile range of (1.10-1.30) while in malignant group the median was 0.26 with interquartile range (0.24-0.28) (Table 1), these results show high statistical significane (P<0.001). In our study using PI cut-off ≤0.28 is reliable for predicting malignancy with sensitivity of 100%, specificity 97.87%, PPV of 75%, NPV of 100% and (P<0.0001) which denotes high statistical significance (Table 2). The results of our study are in agreement with those of Amit et al. [17] who used power Doppler to identify endometrial vessels but then used PI (cut-off PI ≤1.0) as a selection criterion to discriminate between endometrial carcinoma and benign conditions. They found that power Doppler plus PI had higher sensitivity and specificity as compared with measurement of endometrial thickness alone.
Table 3: Receiver-operating characteristic (ROC) curve analysis for prediction of malignant lesions using different parameters measured.
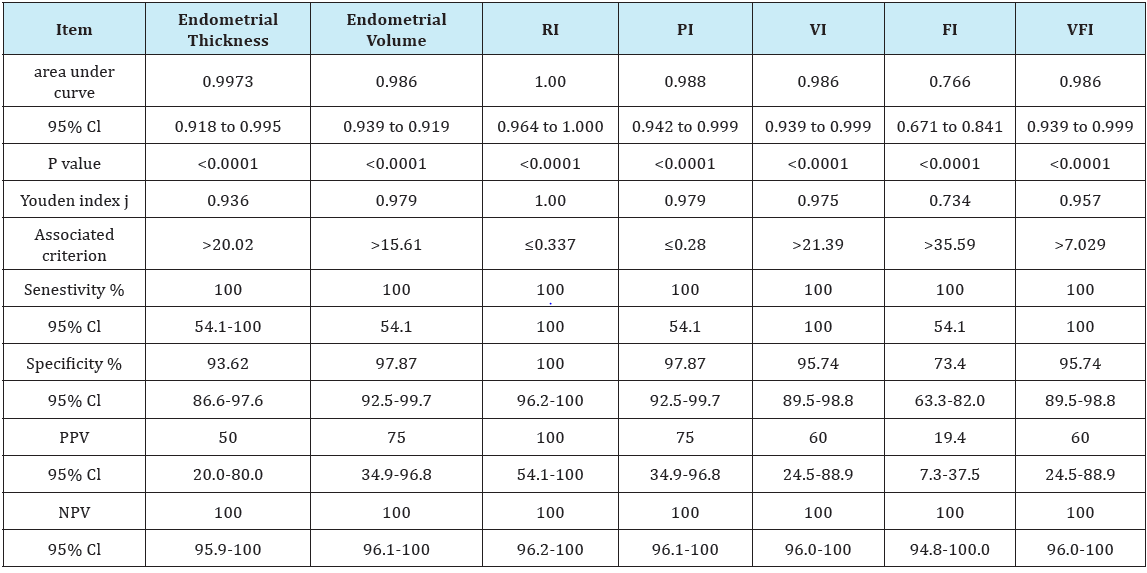
As regards endometrial volume cut-off to predict malignancy, the median endometrial volume (EV) of the whole study population was 4.0 with interquartile range of (2.6-7.4) (Table 1), in nonmalignant group the median was 3.8 with interquartile range of (2.2-5.2) while in malignant group the median was 16.2 with interquartile range (16.1-18.5), these results show high statistical significane (P<0.001) (Table 2). In our study using EV cut-off >15.61 is reliable for predicting malignancy with sensitivity of 100%, specificity 97.87%, PPV of 75%, NPV of 100% and (P <0.0001) which denotes high statistical significance (Table 2). The results of our study are in agreement with those of Gruboeck et al. [18] who analyzed the diagnostic value of endometrial volume in patients with postmenopausal bleeding for diagnosing endometrial cancer. They found that endometrial volume was superior to endometrial thickness measurement for the detection of endometrial cancer.
We are also in agreement with the study of Odeh et al. [19] who reported that the best cut-off for endometrial volume was 3.56mL, with a sensitivity of 93% and a specificity of 36%. The differences in results between studies could be explained as the pathologic group in this study included patients with endometrial cancer and hyperplasia, while in our study; the pathologic group is corresponding to the malignant group which is including patients with endometrial cancer only.
In our results, comparing AUCs of receiver-operating characteristic between endometrial thickness and endometrial volume Table 4 showed no significant difference between them in predicting malignant endometrial lesions (P=0.555). Yet endometrial volume tended to be superior to endometrial thickness as it provides a higher specificity than endometrial thickness (Table 3). An opposite result was reported by Opolskiene et al. [20] who found that measurement of the endometrial volume by three-dimensional sonography did not improve the accuracy of diagnosing endometrial cancer when compared to a conventional two-dimensional measurement of the endometrial thickness.
Regarding the assessment of endometrial vascularization by 3D-PDA, the median vascularity index (VI) of the whole study population was 4.70 with interquartile range of (1.99-7.20) (Table 1), in non-malignant group the median was 4.5 with interquartile range of (1.96-6.10) while in malignant group the median was 34.95 with interquartile range (28.32-35.0), these results show high statistical significane (P< 0.001) (Table 2). In our study using VI cut-off >21.39 is reliable for predicting malignancy with AUC of 0.986, sensitivity of 100%, specificity 95.74%, PPV of 60%, NPV of 100% and (P< 0.0001) which denotes high statistical significance (Table 3). The results of our study are in agreement with those of Alcazar & Galvan [21] who conducted a study to evaluate the role of 3D-PDA to discriminate between benign and malignant endometrial lesions, 99 postmenopausal women with thick endometrium > or =5mm at baseline transvaginal sonography were included in this study, results showed that benign endometrial lesions had mean VI of 2.88 with range of (0.38-5.16) while it was 18.97 with range (13.61-24.33) for malignant endometrium, these results were statistically significant (P<0.001). They also concluded that the best predictor for endometrial cancer was VI with an AUC of 0.90, which is significantly higher than all other parameters. This indicates that endometrial vascularization is increased mainly in cases of adenocarcinoma.
Table 4: Comparison of the areas under the ROC curves (AUCs) associated with various predictors.
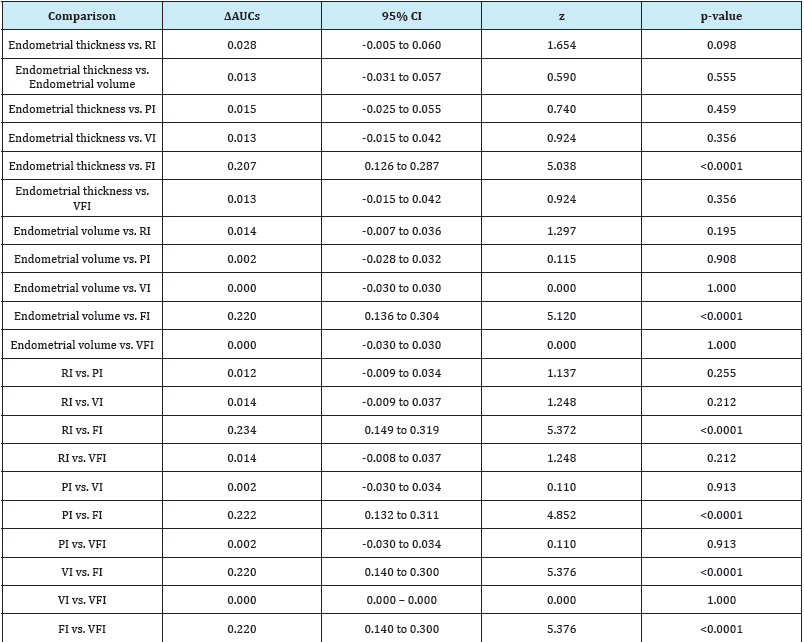
Our results also comes in agreement with more recent study of Hanafi et al. [22] who held a study in Ain Shams University Maternity Hospital on 84 patients with postmenopausal bleeding to determine whether endometrial volume or power Doppler indices measured by 3-dimensional (3D) ultrasound imaging can discriminate between benign and malignant endometrium in women with postmenopausal bleeding and endometrial thickness ≥5mm. In their study, mean VI for benign group was 0.063 with range of (0.039-0.288), while in the malignant group it was 0.687 with range of (0.657-0.687), these results were considered statistically significant (P<0.001). Their endometrial VI cut-off to predict malignancy was >4.0 with AUC of 0.823, sensitivity of 89.29% and specificity of 75%.
The median flow index (FI) of the whole study population was 29.64 with interquartile range of (24.20-38.82) (Table 1), in nonmalignant group the median was 29.35 with interquartile range of (24.16-35.86), while in malignant group the median was 39.96 with interquartile range (36.87-40.0), these results are statistically significant (P=0.029) (Table 2). In our study using FI cut-off >35.59 is reliable for predicting malignancy with AUC of 0.766, sensitivity of 100%, specificity 73.4%, PPV of 19.4%, NPV of 100% and (P<0.0001) which denotes high statistical significance (Table 3). The results of our study are in agreement with those of Mercé et al. [23] who observed that mean endometrial flow index (VI) of endometrial hyperplasia was 18.6 while it was 23.6 for endometrial carcinoma, these results were statistically significant as (P=0.014). Similar results were obtained by Mercé et al. [23] who observed that there is statistical significance (P=0.006) between FI (mean+/- S.D) of endometrial hyperplasia (23.56+/-6.84), and FI (mean+/- S.D) of endometrial carcinoma (29.59+/-8.9).
Our results are also in consistent with the those of of Alcazar & Galvan [21] who showed that benign endometrial lesions had mean FI of 22.96 with range of (11.07-24.55) while it was 29.43 with range of (27.19-31.67) for malignant endometrium, these results were highly statistically significant (P<0.001). We also agree with the results obtained by the study of Hanafi et al. [22] who observed high statistical significance (P<0.001) comparing FI in the benign group [mean: 21.019 with range of (19.231-22.228)] with FI in the malignant group [mean: 25.59 with range of (24.039-28.403)], they also determined that endometrial FI cut-off >23.31 is excellent to predict malignancy with sensitivity of 85.7% and specificity of 98.2%.
The median vascularity flow index (VFI) of the whole study population was 1.35 with interquartile range of (0.71-1.89) (Table 1), in non-malignant group the median was 1.3 with interquartile range of (0.70-1.80), while in malignant group the median was 12.13 with interquartile range (9.34-12.47), these results are statistically significant (P<0.001) (Table 2). In our study using VFI cut-off >7.029 is promising for predicting malignancy with AUC of 0.986, sensitivity of 100%, specificity 95.74%, PPV of 60%, NPV of 100% and (P<0.0001) which denotes high statistical significance (Table 2). The results of our study are in agreement with those of Alcazar et al. [21] who found that benign endometrial lesions had mean VFI of 0.755 with range of (0.02-2.62) while it was 6.00 with range (3.98-8.02) for malignant endometrium, these results were statistically significant (P<0.001).
Similar results were obtained by Mercé et al. [23] who observed that endometrial VFI (Mean+/-S.D) of endometrial hyperplasia was (0.81+/-1.79), while it was (3.59+/-7.94) for endometrial carcinoma, these results denoted high statistical significance as (P<0.001). They also concluded that their best cut-off to predict malignancy among all 3D vascular indices is using endometrial VFI cut-off >2.07. Our results also comes in agreement with the study of Hanafi et al. [22] who observed that mean VFI for benign group was 0.013 with range of (0.007-0.083), while in the malignant group it was 0.193 with range of (0.073-0.522), these results were considered statistically significant (P<0.001). Their endometrial VFI cut-off to predict malignancy was >1.4 which is associated with AUC of 0.823, sensitivity of 89.29% and specificity of 75%.
Our results showed that both endometrial volume and 3D-PDA indices, may discriminate between endometrial cancer and benign conditions as their values were higher in malignant endometrial lesions than those with benign endometrium. In our study AUC of VI, FI and VFI were 0.986, 0.766 and 0.986 respectively (Table 3). Both VI and VFI were equally excellent in predicting malignant endometrial lesions. Our best cut-off values to predict malignant endometrial lesions are VI cut-off of > 21.39 and VFI cut-off of >7.029. In our study, by comparing the AUCs of the receiver operating curve of all the previous parameters, we noted statistical significance (P<0.0001) between endometrial thickness and flow index, endometrial volume and flow index, resistance index and flow index, pulsatility index and flow index, vascularity index with flow index and finally flow index with vascularity flow index (Table 4).
Conclusion
This study showed that the use of three-dimensional sonography and power Doppler angiography can complement the conventional two dimensional ultrasound in assessing the endometrial lesions. It contributes new morphological parameters and non-invasive tumoral angiogenic markers for evaluation of endometrial hyperplastic diseases. The detection of increased endometrial Doppler signals by 3D-PDA may be a possible new ultrasound marker in the diagnosis of endometrial malignancy, and it is worthy of further researches.
References
- O’Connor VM (2003) Heavy menstrual loss. Part 1: is it really heavy loss? Medicine Today 4(4): 51-59.
- Vilos GA, Lefebvre G, Graves GR (2001) Guidelines for the management of abnormal uterine bleeding. Journal of Obstetrics and Gynaecology Canada 23(8): 704-709.
- Speroff L, Fritz MA (2005) Menopause and the perimenopausal transition, clinical endocrinology. In: Speroff L, Fritz MA (Eds.), Clinical gynecologic endocrinology and infertility. (7th edn), Lippincott Williams & Wilkins, London, p. 628.
- Ferrazzi E, Torri V, Trio D, Zannoni E, Filiberto S, et al. (1996) Sonographic endometrial thickness: a useful test to predict atrophy in patients with perimenopausal bleeding. An Italian multicenter study. Ultrasound Obstet Gynecol 7(5): 315-321.
- Smith Bindman R, Kerlikowske K, Feldstein VA, Subak L, Scheidler J, et al. (1998) Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 280: 1510-1517.
- Fleischer AC (1998) Transvaginal sonography of endometrial disorders, an overview. Radiographics 18: 923-930.
- Sawicki V, Spiewankiewicz B, Stelmachów J, Cendrowski K (2005) Color doppler assessment of blood flow in endometrial cancer. Eur J Gynaecol. Oncol 26(3): 279-284.
- Kenneth MN, John SP, Eran BL (2001) Imaging the Endometrium: Disease and Normal Variants. Radiographics 2(6): 1409-1424.
- Englert Golon M, Szpurek D, Moszyński R, Pawlak M, Sajdak S (2006) Clinical value of the measurement of blood flow in uterine arteries and endometrial vessels in women with postmenopausal bleeding using power angio Doppler technique. Ginekol Pol 77(10): 759-763.
- Amit A, Weiner Z, Ganem N, Kerner H, Edwards CL, et al. (2000) The diagnostic value of power Doppler measurements in the endometrium of women with postmenopausal bleeding. Gynecol Oncol 77(2): 243- 247.
- Pairleitner H, Steiner H, Hasenoehrl G, Staudach A (1999) Three dimensional power Doppler sonography: imaging and quantifying blood flow and vascularization. Ultrasound Obstet Gynecol 14(2): 139-143.
- Tekay AH, Järvelä IY, Sladkevicius P, Campbell S, Nargund G (2003) Intraobserver and interobserver variability of ovarian volume, gray-scale and color flow indices obtained using transvaginal three-dimensional power Doppler ultrasonography. Ultrasound Obstet Gynecol 21(3): 277- 282.
- Granberg S, Wikland M, Karlsson B, Norstrom A, Friberg LG (1991) Endometrial thickness as measured by endovaginal ultrasounography for identifying endometrial abnormality. Am J Obstet Gynecol 164(1 Pt 1): 47-52.
- Saha TK, Amer SA, Biss J, Hemlata T, Susan W, et al (2004) The validity of transvaginal ultrasound measurement of endometrial thickness: a comparison of ultrasound measurement with direct anatomical measurement. BJOG 111(12): 1419-1424.
- Tabor A, Watt HC, Wald NJ (2002) Endometrial thickness as a test for endometrial cancer in women with perimenopausal vaginal bleeding. Obstet Gynecol 99(4): 663-670.
- Kupesic S, Kurjak A (2005) Uterine Lesions. Medicinski Glasnik 2(2): 49- 59
- Amit A, Weiner Z, Ganem N, Kerner H, Edwards CL, et al. (2000) The diagnostic value of power Doppler measurements in the endometrium of women with postmenopausal bleeding. Gynecol Oncol 77(2): 243- 247.
- Gruboeck K, Jurkovic D, Lawton F, Savvas M, Tailor A, et al. (1996) The diagnostic value of endometrial thickness and volume measurements by three- dimensional ultrasound in patient with postmenopausal bleeding. Ultrasound Obstet Gynecol 8(4): 272-276.
- Odeh M, Vainerovsky I, Grinin V, Kais M, Ophir E, et al. (2007) Threedimensional endometrial volume and 3-dimensional power Doppler analysis in predicting endometrial carcinoma and hyperplasia. Gynecol Oncol 106(2): 348-353.
- Opolskiene G, Sladkevicius P, Jokubkiene L, Valentin L (2010) Threedimensional ultrasound imaging for discrimination between benign and malignant endometrium in women with postmenopausal bleeding and sonographic endometrial thickness of at least 4.5 mm. Ultrasound Obstet Gynecol 35(1): 94-102.
- Alcazar JL, Galvan R (2009) Three-dimensional power Doppler ultrasound scanning for the prediction of endometrial cancer in women with postmenopausal bleeding and thickened endometrium. Am J Obstet Gynecol 200(1): 44.
- Hanafi S, Abou gabal A, Akl S, Abd el baset H (2014) Value of three dimensional power Doppler ultrasound in prediction of endometrial carcinoma in patients with postmenopausal bleeding. J Turk Ger Gynecol Assoc 15(2): 78-81.
- Merce LT, Alcazar JL, Lopez C, Iglesias E, Bau S, et al (2007) Clinical usefulness of 3-dimensional sonography and power Doppler angiography for diagnosis of endometrial carcinoma. J Ultrasound Med 26(10): 1279-1287.
© 2017 Ahmed Sherif, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






