- Submissions

Full Text
Integrative Journal of Conference Proceedings
A New Facet of Symmetry in Chemistry and Biochemistry
Iga DP1,2*, Popescu D3 and Niculescu VIR4
1University of Bucharest, former CI Parhon, Romania
2University of Oradea, B-dul Armata Romana, Romania
3Gh Mihoc-Caius Iacob Institute of Mathematical Statistics and Applied Mathematics of Romania Academy, Romania
4Institut de Recherche et Development pour les Lasers, Plasma et Physique de la Radiation, Romania
*Corresponding author: Iga DP, University of Bucharest, former CI Parhon, Bucharest, University of Oradea, B-dul Armata Romana, Oradea, Romania
Submission: April 09, 2021;Published: May 11, 2021

Volume2 Issue5May, 2021
Abstract
The phenomenon described below concerns molecules having a content of chirality. A new type of symmetry, complementary to the classical symmetry characterized by a mirror plane of symmetry is revealed. As the latter generates an enantiomeric image, the plane of symmetry of this new type of symmetry generates an identical one. The rank of this new plane of symmetry is lower than mirror plane of symmetry. Arguments from chemistry, biology and practical life are brought in support of this new facet of chiral compounds.
Introduction
Detection and measurement of the physical magnitude called optical activity became a
current analysis in chemical laboratories in the first quarter of the eighteenth century [1-4].
An exceptional experiment based on measurement of optical activity was made by Pasteur
L [5]. He worked on a specimen of tartaric acid devoid of optical activity (racemic or paratartaric
acid) that had been prepared at industrial level by Kestner (1822), an Alsatian
manufacturer Kendall J [6], Derewenda ZS [7]. Dextro-tartaric acid had been discovered by
Scheele (1770) in the sediment deposited in the vats during the grape juice fermentation [8,9].
Kestner’s specimen had the same chemical properties as tartaric acid discovered by Scheele.
However, some physical properties (solubility in water, crystalline form, optical activity, etc.)
were different [6]. Pasteur prepared the double salt of sodium-ammonium of para-tartaric
acid and then crystallized it. He noticed two types of crystals, that were enantiomorphic with
one another. Pasteur separated the two types of crystals and found out that their aqueous
solutions were dextrorotary and levorotary, respectively. Consequently, the so-called paratartaric
acid was in fact a racemic mixture, (±)-tartaric acid. Another isomer, devoid of optical
activity and not cleavable by any chemical or biological method, was discovered also by
Pasteur (1853) and called meso-tartaric acid [6].
Van’t Hoff JH [10] & LeBel JA [11] signed the birth certificate of stereochemistry by their
hypothesis concerning tetrahedral C atom. However, at that time no scientist in the world
could rationally associate structural models with the two enantiomers [12]. In fact, the
discovery of Pasteur increased the dilemma of representation, i.e., the relationship between
a sample of an optically active compound and the unique, characteristic, structural model
possibly assigned to it. This dilemma was solved by X-ray diffraction, i. e., zirconium Kα rays,
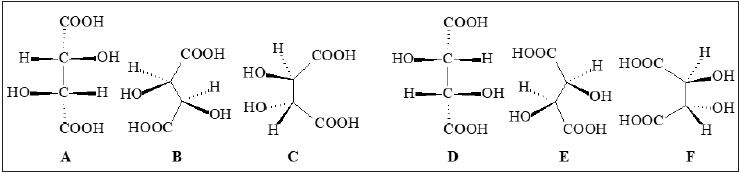
by sodium rubidium tartrate of the dextrorotary species, and the obtained model Figure.
1A-C was assigned to (+)-tartaric acid [13]. By an impressive coincidence, this configuration
of (+)-tartaric acid had been hypothetically attributed by Fischer E [14]. Configuration of
chiral centers of (–)-tartaric acid (Figure 1D-F) became also known, by the virtue of the law
of enantiomorphism. The nomenclature of the two enantiomers became L-(+)-tartaric and
D-(–)-tartaric acid, according to the recommendations of Fischer E [15-17], Rosanoff MA
[18] & Wohl A, et al. [19]. The isomers of tartaric acid were structurally correlated with the
isomers of glyceraldehyde and other monosaccharides as well as with other chiral compounds
[19,12,20].
Figure 1: Structural models assigned by Bijvoet JM, et al. [13] to L-(+)-tartaric acid (A, B, C). Configuration of chiral centers of D-(–)-tartaric acid (D, E, F) became known by the virtue of the law of enantiomorphism.
In fact, no other scientist contributed as much as E. Fischer
to the transformation of hypothesis of van’t Hoff and LeBel in a
veritable theory. E. Fischer used a quasi-unique technique, when he
elaborated the famous suite of papers concerning the elucidation of
monosaccharides structure. His technique consisted in the so-called
equalization-deequalization of the two ends of monosaccharides,
either by reduction (Na/Hg) to hexitols or by oxidation (nitric
acid) to aldaric acids. Then he seemingly returned to the initial
compound, also by redox reactions, with the hypothesis in his mind
that the two ends have a similar chemical reactivity [21-24].
By this system of reactions, he found out three types of linear
aldohexoses:
A. Monosaccharides that reproduced themselves, e.g., D- and
L-mannose, D- and L-idose;
B. Monosacharides which besides themselves produced
another isomer, e.g., D-glucose produced besides itself a new
sugar called L-gulose [25];
C. Monosaccharides that produced a racemic mixture, as if
the inner enantiomorphy of their polyol or aldaric acid became
externalized in the products, e.g., D-galactose led to D- and
L-galactose. Concerning the compounds with identical ends,
those produced by (i) and (ii) monosaccharides were optically
active, while hexitols and aldaric acids produced by (iii)
monosaccharides possessed a mirror plane of symmetry and
consequently were optically inactive (meso). All three types of
monosaccharides, (i)-(iii), were isomers, and their derivatives
with identical ends, hexitols and aldaric acids constituted other
two groups of isomers.
Meso compounds (meso-tartaric acid, erythritol, galactitol,
allitol, galactaric and allaric acid, etc.) are devoid of optical activity.
These compounds possess an even number of atoms in their
molecule, they are homodimers. They are characterized by a mirror
plane of symmetry drawn between atoms. The tentative of E. Fischer
to expand the concept of meso to some heterodimers, e.g., xylitol,
ribitol (adonitol), etc., remained a definitive choice in chemical
literature. (According to other Fischer’s papers concerning chemical
synthesis of monosaccharides, as well as to some biosynthesis
schemes [26], xylitol and ribitol can be considered as products of
reductive dimerization of glycol aldehyde and glyceraldehyde).
Fischer found out that both pentitols are devoid of optical activity
[27,28], hence they have symmetrical molecules. The mirror plane
of symmetry of some heterodimers contains a series of atoms [C-
3, H and OH in case of xylitol and ribitol, C-3 and two H in case
of 3-deoxyxylitol [29,30] and 3-deoxyribitol [31], etc.]. One can
inferr the following rule: all atoms situated within mirror plane of
symmetry equally contribute to the chirality of the two halves of the
molecule. Hence, we judge heterodimers possessing a mirror plane
of symmetry in an idealistic manner: the atoms cut by the mirror
plane of symmetry are ignored (or imaginarily eliminated) and
what remains is formed of two halves, and involves an even number
of C, in fact an even number of atoms. With this amendment, meso
compounds can be defined as being formed of two enantiomeric
chiral halves. Meso compounds devoid of a mirror plane of
symmetry are rigorously formed of an even number of atoms and
they are analyzed by Cahn-Ingold-Prelog rules. The result should
be an equal number of R and S asymmetric carbons; meso-tartaric
acid is (2R,3S or 2S,3R). According to Kelvin and Prelog rules, meso
compounds are heterochiral (Kelvin WT [32], Prelog V [33], Cronin
J, et al. [34]).
Structural analysis of dextro- and levo-tartaric acid Figure 1
indicates that their molecule is formed of two prochiral identical
halves: they are (2R,3R) and (2S,3S), respectively. The same
situation is met with D- and L-threitol, D- and L-mannitol, D- and
L-iditol, etc. This phenomenon has been disclosed for the first
time by Jaeger FM [35] in describing crystals formation by the
aggregation of smaller ones (rudimentary or embryonic), to form
grown, mature crystals. He used the term twin (or twinning),
especially when two such components are united. We have adopted
this term and adapted it for chemical and biochemical purposes.
We have patterned it as chitwin (chi from chiral plus twin), and
we have called the constituents of this group chitwin compounds
or chitmers [36-38]. They have been defined as chiral molecules
formed of two identical halves. To chitmers possessing an odd
number of atoms one applies the same reasoning as for meso
ones. Chitmers whose meso isomers are devoid of a mirror plane
of symmetry are analyzed by Cahn-Ingold-Prelog rules: the result
should be two identical sets of asymmetric carbons.
A chiral molecule can be twinned outwardly, i.e. a simple
multiplication, or internally, and in this case a chitwin molecule
could be produced. Moreover, in order to describe the symmetry
of crystalline systems, Jaeger used the term twinning-plane. This reasoning discloses a new type of symmetry at molecular level, and
even at macrocosmic one. As mirror plane of symmetry involves
two enantiomeric halves, the twinning-plane (we have called it
chitwin¬ plane) associates two prochiral identical halves. The
chitwin plane has a lower rank than mirror plane of symmetry: LAnd
D-arabinitol are not chitwin but simply chiral. However 3-ketoand
3-deoxy-arabinitol are chitwin. This concept is supported by
many hundreds of natural and artificial compounds, about an
order of magnitude bigger than the number of meso compounds.
And their multitude is increasing every day. This concept applies
especially in chemistry and biology but also in practical life. Many
decades after Jaeger’s book, they were called C2 symmetrical
[39-41]. Chitwin molecules possess a distinctive structure, being
formed of two sets of identical chiral carbons and two sets of
identical chemical functions. They are internally homochiral and
they are C2 symmetrical since they are chitwin. The principle used
by E. Fischer to expand the subgroup of homodimeric compounds,
also works for chitwin ones although in a more limited manner.
In the decades after E. Fischer, chitmers were found among many
other families of compounds: amino acids and their derivatives
[42,43], carotenoids Iga DP [38], lignans, cyclobutane derivatives,
phenolic compounds, alkaloids, terpenoides, lipids, coenzymes
based on nucleosides or on cysteine and cysteamine, in oxidized
state, homodimeric proteins, palindromes [36,37]. At the same
time, chitwin phenomenon has been adopted as a principle of
chemical synthesis [44].
References
- Malus EL (1809) On a property of reflected light. Mém Soc Arc 2: 143-158.
- Arago FJD (1811) Thesis on a remarkable modification experienced by light rays as they pass through certain diaphanous optical bodies. Mém Inst 12: 93-134.
- Biot JB (1815) Successive polarization phenomena observed in homogeneous fluids. Bull Sci Soc Philom 190-192.
- Nicol W (1829) On a method of so far increasing the divergency of the two rays in calcareous spar that only one image may be seen at a time. Edinburgh New Phil J 6: 83-84.
- Pasteur L (1848) Brief on the relation which may exist between the crystalline form and the chemical composition, and on the cause of the rotational polarization. Compt Rend 26: 535-538.
- Kendall J (1953) Great discoveries by young chemists, Th Y Growell Company, New York, USA.
- Derewenda ZS (2008) On wine, chirality and crystalography. Acta Cryst A 64(Pt 1): 246-258.
- Wisniak J (2009) Carl Wilhelm Scheele. Rev CENIC Cienc Quim 40: 165-173.
- Svedberg GA (2012) Tribute to the memory of carl Wilhelm Scheele. Anna Lindberg, Kaigan AB (Eds.), Stockholm, Sweden.
- van't Hoff JH (1874) A suggestion looking to the extension into space of the structural formulas at present used in chemistry: And a note upon the relation between the optical activity the chemical constitution of organic compounds. Arch Neerland Sci Nat 9: 445-454.
- Le Bel JA (1874) On the relations which exist between the atomic formulas of organic bodies and the rotary power of their solutions. Bull Soc Chim 22: 337-347.
- Hoffmann R, Laszlo P (1991) Representation in Chemistry. Angew Chem 30(1): 1-16.
- Bijvoet JM, Peerdemann AF, Van Bommel AJ (1951) Determination of the absolute configuration of optically active compounds by means of X-rays. Nature 168: 271-272.
- Fischer E (1896) Configuration of tartaric acid. Ber Deut Chem Ges 29(2): 1377-1383.
- Fischer E (1891) About d and i mannosugar acid. Ber Deut Chem Ges 24(1): 539-546.
- Fischer E (1891) About the configuration of grape sugar and its isomers. Ber Deut Chem Ges 24(1): 1836-1845.
- Fischer E (1891) About the configuration of grape sugar and its isomers II. Ber Deut Chem Ges 24(2): 2683-2687.
- Rosanoff MA (1906) On fischer's classification of stereo-isomers. J Am Chem Soc 28(1): 114-121.
- Wohl A, Momber Fr (1917) The steric relationship between glyceraldehyde and tartaric acid. Ber deut chem Ges 50(1): 455-462.
- Iga DP (2018) Basic principles of the strategy concerning the elucidation of configuration of chiral centers of linear isomeric aldohexoses. Foundations of Chemistry 20(1): 31-41.
- Fischer E, Hirschberger J (1888) Ueber Mannose. Ber Deut Chem Ges 21(1): 1805-1809.
- Fischer E, Hirschberger J (1889) Ueber mannose II. Ber Deut Chem Ges 22(1): 365-376.
- Fischer E (1889) Reduction of acids of the sugar group. Ber Deut Chem Ges 22(2): 2204-2209.
- Fischer E, Hertz J (1892) Reduction of mucic acid. Ber Deut Chem Ges 25(1): 1247-1261.
- Fischer E, Piloty O (1891) Reduction of the sugar acid. Ber Deut Chem Ges 24(1): 521-528.
- Metzler DE, Metzler CM (2003) Biochemistry: The chemical reactions of living cells. Elsevier, Amsterdam, Netherlands.
- Fischer E, Stahel R (1891) To the knowledge of xylose. Ber Deut Chem Ges 24(1): 528-539.
- Fischer E (1893) About Adonit, a new Pentit. Ber Deut Chem Ges 26(1): 633-639.
- Anderson PJ (1965) Oxidation of 3-deoxyxylitol by L-ldltol dehydrogenase. Biochim Biophys Acta 110(3): 627-629.
- Stankovic E, Bilik V, Fedoronko M, König Stein J (1975) Reactions of saccharides catalyzed by molybdate ions XIV epimerization of pentuloses. Chem zvesti 29(5): 685-689.
- Oka J, Ueda K, Hayaishi O, Komura H, Nakanishi K (1984) ADP-ribosyl protein lyase. Purification, properties, and identification of the product. J Biol Chem 259(2): 986-995.
- Kelvin WT (1904) Baltimore lectures on molecular dynamics and the wave theory of light, CJ Clay, London, pp. 602-642.
- Prelog V (2006) Chirality in chemistry. Nobel Lecture, Croatica Chemica Acta, The Nobel Foundation 1975 79(3): XLIX-LVII.
- Cronin J, Reisse J (2005) 3 Chirality and the origin of homochirality. In: (Gargaud M, Barbier B, Martin, H, Reisse J (Eds.), Lectures in Astrobiology, Springer-Verlag, London 1: 73-114.
- Jaeger FM (1917) Lectures on the principle of symmetry and its applications in all natural sciences. Elsevier Publishing Co., Amsterdam, Netherlands.
- Iga DP (2018) Chitwin compounds: A new revelation of chemistry and biology. Chemistry Research Journal 3(4): 63-79.
- Iga DP (2020) A new kind of symmetry in chemistry and biology and a virtual mirror intrinsic to vegetable tissues evidenced by comparative structural analysis of dochi compounds. Chemistry Research Journal 5(1): 71-91.
- Iga DP (2021) Carotenoid structures, an illustration of a new kind of symmetry in chemistry. Chemistry Research Journal 6(1): 20-48.
- Whitesell JK (1989) C2 symmetry and asymmetric induction. Chemical Reviews 89(7): 1581-1590.
- Ghosh AK, Mathivanan P, Cappiello J (1998) C2-Symmetric chiral bis(oxazoline)-metal complexes in catalytic asymmetric synthesis. Tetrahedron Asymm 9(1): 1-45.
- Pfaltz A, Drury WJ (2004) Design of chiral ligands for asymmetric catalysis: From C2-symmetric P,P- and N,N-ligands to sterically and electronically nonsymmetrical P,N-ligands. Proc Natl Acad Sci USA 101(16): 5723-5726.
- Vickery HB (1957) Assignment of D and L prefixes to the tartaric acids. J Chem Educ 34(7): 339-341.
- Borthwick AD (2012) 2,5-diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem Rev 112(7): 3641-3716.
- Berube G (2006) Natural and synthetic biologically active dimeric molecules: Anticancer agents, anti-HIV agents, steroid derivatives and opioid antagonists. Curr Med Chem 13(2): 131-154.
© 2021 Iga DP. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






