- Submissions

Full Text
Integrative Journal of Conference Proceedings
Dry Eye Disease: A Comprehensive Review
Manish Dahal1, Raju Kaiti1*, Sabina Nepal2, Ranjila Shyangbo3, Grishma Dahal2 and Rekha Ghimire2
1Consultant Optometrist, Nepal Eye Hospital
2BOptom, Class of 2023, National academy of medical sciences, Nepal
3BOptom, Class of 2022, National academy of medical sciences, Nepal
*Corresponding author: Raju Kaiti, MOptom, Consultant Optometrist, Nepal eye hospital, Lecturer, National academy of medical sciences, Nepal
Submission: August 14, 2020;Published: September 15, 2020

Volume2 Issue3September, 2020
Abstract
Dry eye is ubiquitous but under diagnosed ocular morbidity. Many people didn’t recognize or refuse this ocular morbidity due to inadequate knowledge, but now our understanding of DED has improved dramatically over the last 20 years with advancements in research .Dry eye disease is multifactorial disease of tear film and ocular surface that result in discomfort, visual disturbance and tear film instability with possible damage to ocular surface. Tear film is a complex mixture of substance secreted from the multiple sources on the ocular surface, including lacrimal gland, meibomian glands and goblet cells. As the population ages, the prevalence of dry eye is likely to increase, yet the condition is often underrecognized and undertreated. This comprehensive review article describes our current knowledge and understanding of the causes and interprets the detail information on definition, prevalence, etiology, classification, diagnostic test, and method of management of dry eye disease. This paper also discusses on ongoing researches, current challenge and future directions for advancing knowledge and treatment of the condition. Via this comprehensive review, in the light of our current understanding of DED, we aim to provide awareness among the patients, health care professionals, researchers, and especially among them who are at major risk for developing DED about diagnosis and treatment of DED and recent developments and future challenges in management of dry eye disease. Beside these, we have also stressed on lifestyle changes and dietary behaviors that may affect the tear dynamics.
Keyword: Dry eye disease;Keratoconjunctivitis sicca;Ocular morbidities;Treatment
Introduction
Dry eye disease (DED) is one of the ubiquitous and under-diagnosis ocular morbidities experienced by every eye care practitioner. National Eye Institute(NEI) in 1995 defined DED as a disorder of tear film due to reduced tear production or excessive tear evaporation, which causes damage to the inter-palpebral ocular surface and is associated with symptoms of ocular discomfort and/visual symptoms. In 2007 International Dry Eye Workshop (DEWS) defined DED as a “multifactorial disease of tears and ocular surface that results in symptoms of discomfort, visual disturbance and tear film instability with potential damage to ocular surface. It is accompanied by increased osmolality of the tear film and inflammation of the ocular surface [1]. DEWS was more on scientific knowledge and understanding and is based on aetiology, mechanism, and severity of the disease. In 2011, International Workshop on Meibomian Gland Dysfunction(MGD) was formed where wider coverage on MGD was done [2]. MGD is one of the leading causes of dry eyes worldwide. Our understanding of DED has improved dramatically over the last 20 years with advancements in research-some of which are listed on Table 1. Various research has been conducted regarding the dry eye disease, recent studies show that that dry eye seems to be caused by inflammation mediated by T-cell lymphocytes [3] but it is still a topic of controversy. The role of anti-inflammatories in dry eye management have however augmented the inflammatory nature of DED. DES is also called keratoconjunctivitis Sicca (KCS), keratitis sicca, sicca syndrome, xerophthalmia, Dry Eye Disease (DED), Ocular Surface Disease (OSD), or Dysfunctional Tear Syndrome (DTS), or simply dry eyes [1]. Nowadays, research on dry eyes are particularly focused on eliciting its pathogenesis, new advancements in its management and implication of dry eye in visual function and quality [4].
Table 1: Prevalence of dry eye in different population-based studies in different parts of the World.
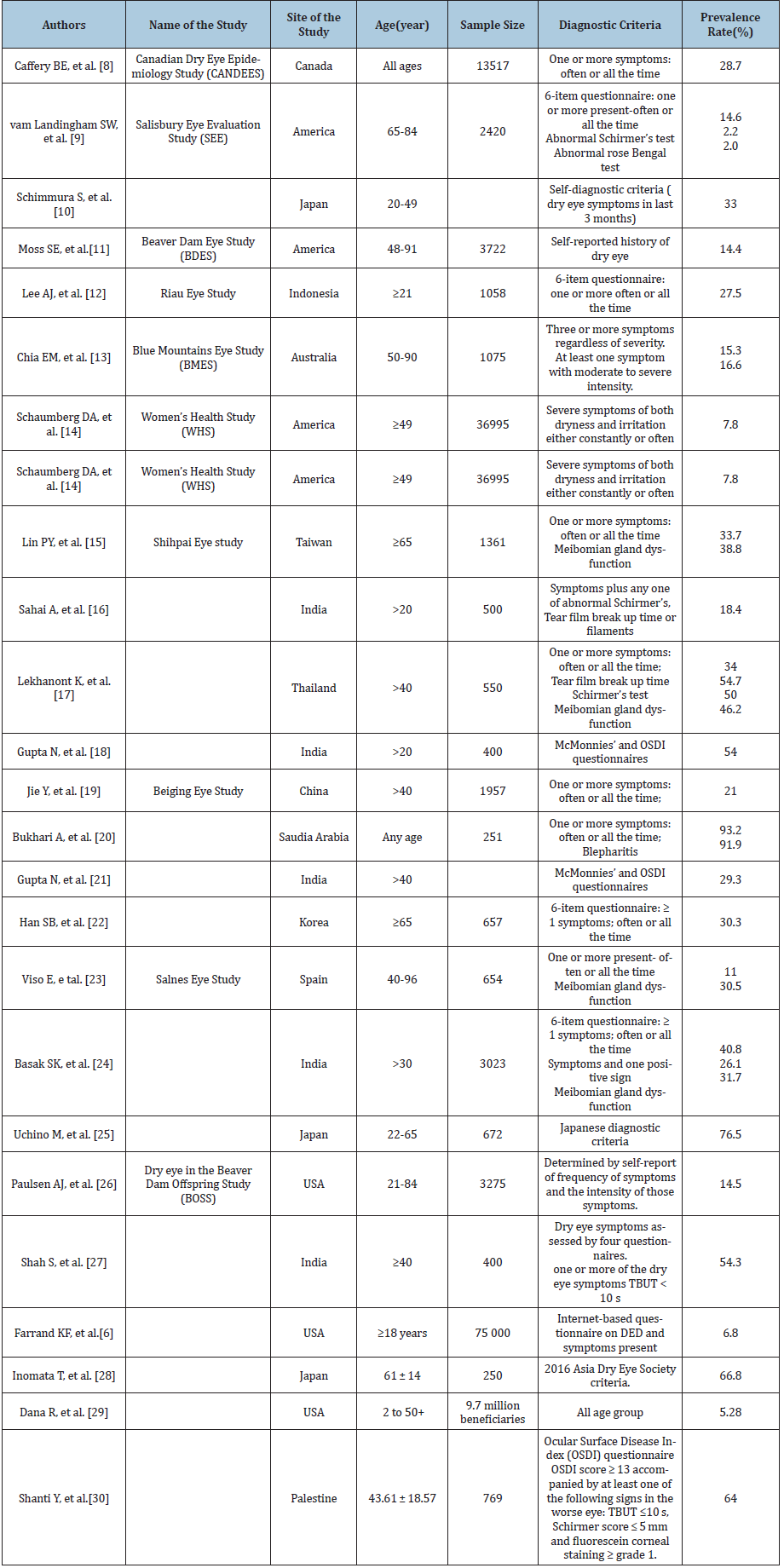
Prevalence of Dry Eye
Prevalence of disease varies with operational definition of dry eye used and characteristics of the population studied. Prevalence of dry eye ranges from 5-50% [5]. It has been meta-analyzed that prevalence of DED increases with age and is higher in females than in male, although the difference becomes significant only with age. Based on data from the National Health and Wellness Survey, 6.8% of the United States adult population (approximately 16.4 million people) have been diagnosed with DED6. The prevalence increased with age (2.7 % in those 18 to 34 years old versus 18.6% in those ≥75 years old) and was higher in women than men (8.8 versus 4.5 percent). Prevalence was not affected by education or location of residence [5,6].
Based on the data from research conducted on Nepal Eye hospital (among the 100 patients) revealed that female preponderance in the patients with a ratio of 2.6:1, The number of premenopausal females outweighed the number of postmenopausal in this study (53 vs 20). Most affected age group was between 30 to 40 years (29%). Amongst the modifiable attributable risk factors were urban residence (69%), non-vegetarians consuming red meat primarily (53%), exposure to computers and air conditioner (39%). Systemic diseases like diabetes mellitus, hypertension, and arthritis make up the non-modifiable risk factor [7]. Table 1 shows the prevalence of dry eye in different population-based studies in different parts of the World [8-30].
Tear Film
The tear film is a complex mixture of substances secreted from multiple sources on the ocular surface, including the lacrimal gland, the accessory lacrimal glands, the meibomian glands, and the goblet cells. It is composed of 3 layers i.e. lipid, aqueous and mucin layer whereas all layers has its own importance to maintain integrity of tear film [31].
Physical properties
1. Thickness :7-8μm
2. Volume: 4-13μl
3. Rate of secretion: 1.2μl per min
4. Turnover rate: 18% per min
5. Refractive index: 1.357
6. Osmotic pressure: 0.90 to 0.95% NACL solution, 350 at the limbus; 300 at the Centre
7. PO2: 40-160 mmHg
Composition
Lipid layer is the outermost oily layer derived from Meibomian, Zeiss and Moll glands. Its thickness is about 0.1 to 0.2μm and contain lipids of low polarity which prevent overflow of tears and retard their evaporation. Aqueous layer is a middle layer derived from the main Lacrimal gland and the accessory glands of Krause and wolfring. Its thickness is about 7μm and constitute of aqueous solution of low viscosity, ions of inorganic salt, glucose, urease and various biopolymers such as protein, enzymes, lysozymes etc. which provide atmospheric oxygen to epithelium, also provide antibacterial, anti-adhesive and lubricating properties. The innermost layer is mucin layer derived from goblet cell, crypts of Henle and gland of Manz. Its thickness is about only 0.02 to 0.04μm and consists of highly hydrated and semi-solid mucus made of glycoprotein that helps the eye to remain moist and lubricate. Also protect epithelium of cornea and conjunctiva from abrasion [31,32] Problem with any of these sources of tear film may lead to instability of tear film and cause dry eye.
Etiology, Causes and Risk Factor
Etiology of dry eye disease varies from person to person. Causes for DED include decreased tear production, excessive tear evaporation, and abnormality in the production of mucus or lipids of tear layer [33,34]. Recent studies also show that dry eye diseases are found to be associated with auto-immune diseases like Sjogren’s syndrome and Rheumatoid arthritis [3,34]. Appreciation of the role of inflammation in DED was one of the most important factors that aided in the understanding and treatment of DED. The findings of the association of inflammation with reduced tear secretion and subsequent damage to the ocular surface led to the proposal of a unified concept of DED [34,35].
Other factors that precipitate and/or exacerbate DED include long-term use of contact lens , refractive surgeries such as laser-assisted in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK) [36,37] hypovitaminosis A [38], smoking [39], extended visual tasking during computer use, television watching and prolonged reading provoke symptoms of dry eye [40]. Certain medication such as antihistamines, decongestants, blood pressure medications and antidepressants induce DED [41,42]. Environmental factors like exposure of the eye towards pollution, smoke, wind and dry climates can increase tear evaporation resulting in DED [43].
Classification
The first comprehensive classification of dry eye was published in 1995 on the basis of consensus from the NEI/Industry working group on Clinical Trials in Dry Eye [44] Dry eye was defined as “a disorder of the tear film due to tear deficiency or excessive tear evaporation” suggesting that dry eye caused either by tear deficiency or excessive evaporation. In the report, dry eye was divided into 2 primary categories; tear-deficient and evaporative. These two subgroups were further sub-classified according to a range of intrinsic and extrinsic etiological factors. Chinese scholars proposed their dry eye classification in 2004 [45] It proposed a method based on the structure of tear film and tear dynamics. Dry eye was divided into five types, including lipid deficient dry eye (evaporative dry eye), aqueous deficient dry eye, mucin deficient dry eye, abnormal tear dynamics dry eye, and mixed dry eye.
Table 2: Dry eye disease severity grading scheme presented in TFOS DEWS report in 2007.
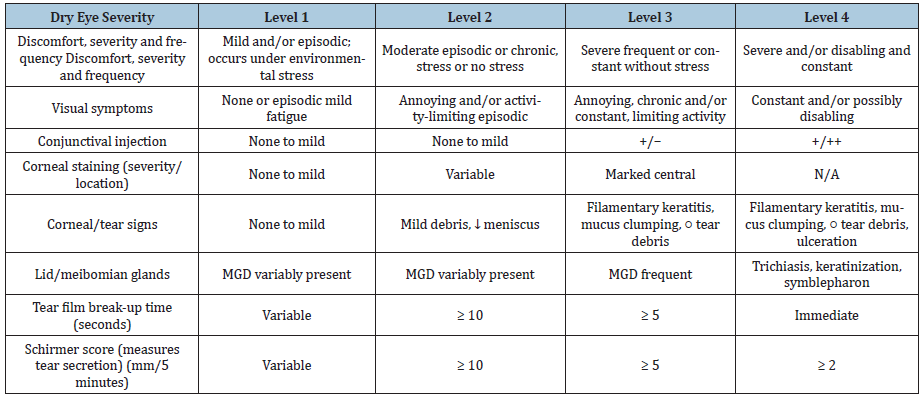
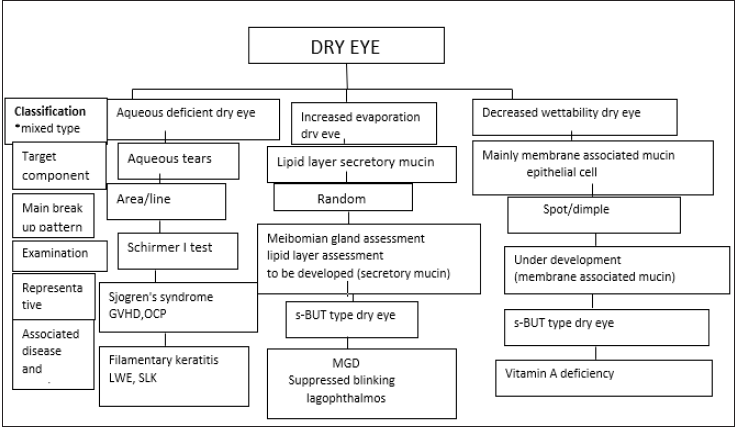
In 2005 Murube J, et al. [46] published an article entitled “The triple classification of dry eye for practical use”. His classification consisted of the three following aspects: etiopathogenesis, affected glands/tissues, and grade of severity. In relation to etiopathogenesis, dry eye was classified into the following 10 subcategories: agerelated, hormonal, pharmacologic, immunopathic, hyponutritional, dysgenetic, adenitic, traumatic, neurologic, and tantalic. In a classification according to the affected glands/tissues, there were the following five subcategories: aqueous deficient, lipid deficient, mucin-deficient, epitheliopathic, and nonocular exocrine-deficient. Later in 2007, DEWS published a report and aqueous deficient dry eye was further classified into Sjogren and non-Sjogren categories (Figure 1). Evaporative dry eye was sub classified into intrinsic and extrinsic categories, and they were further classified according to etiological factors and severity of dry eye [1] The classification system also took its place in the recent Tear Film and Ocular Surface (TFOS) DEWS II report with some modification [47]. The newly proposed classification scheme considers the cases where patients exhibit dry eye symptoms without evidence of obvious signs, or present with marked signs, but lack of dry eye symptoms. The former includes cases with neuropathic pain where the somatosensory system is affected, and the latter is related to the reduced corneal sensitivity (neurotrophic condition) (Figure 2). Eyes with both signs and symptoms were classified into either aqueous deficient or evaporative dry eyes. Table 2 shows the dry eye disease severity grading scheme presented in the TFOS DEWS report in 2007. In 2019, new dry eye classification based on the tear film-oriented diagnosis concept was proposed by Asian Dry Eye Society. They categorized dry eye disease into three types according to the tear film abnormality or epithelial surface abnormality (Figure 3). Their classification included three types of dry eye: increased evaporation, aqueous-deficient, and decreased wettability [48].
Figure 1: Major etiological cause of dry eye in TFOS DEWS report in 2007.

Figure 2: Classification of dry eye disease in TFOS DEWS II.
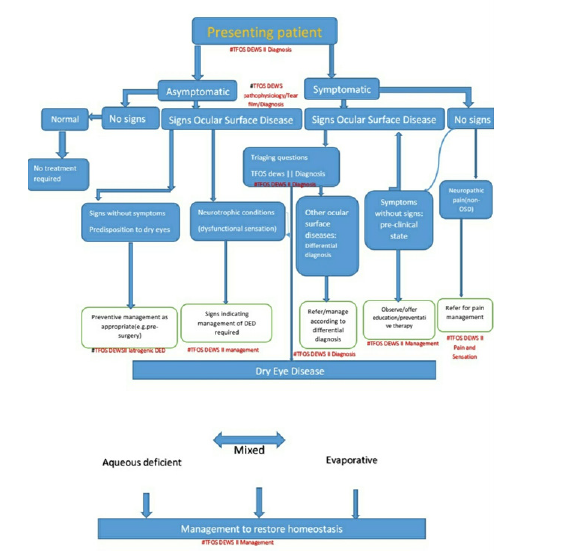
Figure 3: New dry eye disease classification by the Asia dry eye society.
Symptoms
Symptoms of DED usually occur bilaterally, may include;
1. Photophobia [49]
2. Foreign body sensation [49]
3. Red eye [49,50]
4. Stringy mucus in or around eyes [50]
5. Blurred vision or eye fatigue [51]
6. Burning and a sandy-gritty eye irritation [52]
7. Watery eyes this is because dryness on the eye’s surface sometimes will over-stimulate production of the watery component of your tears as a protective mechanism. But this “reflex tearing” does not stay on the eye long enough to correct the underlying dry eye condition [50,53]
Diagnosis
Diagnosis of Dry Eye Disease (DED) is made by combining information obtained from the physical examination and performing diagnostic tests. The clinical manifestations of DED can be highly variable; hence the diagnosis is often based on a combination of symptoms, signs, and clinical tests, given that any one of these alone would miss a significant number of patients. The role of the Diagnostic Methodology Subcommittee of the Dry Eye Workshop was to identify tests used to screen, diagnose and monitor dry eye disease, to establish criteria for test performance, and to consider the utility of tests in a variety of clinical settings [54]. Various diagnosis tests have been added in order to diagnose DED. These include:
Schirmer test
It is the most common test for diagnosis of DED which measures total secretion of tears. It is performed with the help of a 5*35mm strips of Whatman-41 filter paper which is folded 5mm from one end and kept at the junction of 1/3rdfrom lateral canthus and 2/3rdfrom medial canthus in lower fornix. Patients are asked to move up and not to blink their eyes then after 5mins wetting of the filter paper strip from the bent end is measured. Value above 15mm is said to be normal whereas below 15mm are considered as dry eye. There are 3 variations of the Schirmer test [55,56].
A. Schirmer test I: Measures total tear secretion (basal and reflex) along with or without topical anesthesia [57].
B. Schirmer test II: measure reflex secretion only and involves nasal stimulation following insertion of the strip [58].
C. Schirmer test III: measure reflex secretion by allowing the patient to look directly into the sun [55].
Tear film break-up time [TBUT]
It is the interval between a complete blink and appearance of the first randomly distributed dry spot on the cornea . BUT is an indicator of inadequate mucus component in tear film. It is noted after instillation of drop from fluorescein strip and examination in cobalt-blue light in slit-lamp. Normal values range from 15 to 35sec, value less 10sec imply tear film instability [59,60]. TBUT can also be measured without the addition of fluorescein to the tear film, this is called non-invasive BUT (NIBUT). It uses a grid or other patterns directed on the precorneal tear film for observation of image distortion and time from opening the eyes to the first sign of image distortion is measured in seconds [61].
Tear meniscus height (meniscometry)
The tear meniscus height can be used to estimate tear volume. Under this, the height of tear meniscus observed during slit lamp examination. Tear meniscus is a continuous, full and slightly concave meniscus formed by tear between eyelid margin and inferior bulbar conjunctiva where the lid touches the globe.Normal tear meniscus height is 0.3mm, less than 0.25mm is suggestive of dry eye [62].
Rose Bengal staining
It is a very useful test for detecting even mild cases of KCS. Tear staining was assessed by successive timed Schirmer strip collections after instillation of 1% RB into the lower fornix. Depending upon the severity of KCA three staining patterns A, B and C are seen [63].
Pattern C: represent mild or early cases with fine punctate stains in the interpalpebral area
Pattern B: represent mild case with extensive staining Pattern A: represent severe cases with confluent staining of conjunctiva and cornea
Lissamine Green staining
Lissamine Green staining has almost identical properties with Rose Bengal staining and stain dead, degenerated and mucus cells. But it seems to be more irritating than Rose Bengal staining [64].
Tear lactoferrin test
Lactoferrin is an antiviral, antibacterial iron-binding glycoprotein that is vital to tear production. It is a mucus-specific anti-inflammatory molecule which is produced by acinar cells in the lacrimal gland. The tear lactoferrin test can be used to diagnose the quality of tears/mucin, DED, and hyperosmolarity. A drop of tear fluid is collected from the lower eyelid and then placed onto a microscope slide and allowed to dry by evaporation. Different forms of branching crystallization patterns are observed and classified. The test diagnoses dry eyes on the basis of the forming patterns [54,65].
Tear lysozyme level
It is a qualitative and quantitative test to measure tear lysozyme level. In the early stage of dry eye tear lysozyme level is decreased [66].
Tear film osmolarity
Osmolarity testing measures the concentration of solutes in the tear film where higher levels or hypertonic condition indicate a reduced aqueous component, either by increased evaporation or reduced aqueous secretion. Normal tear osmolarity is isotonic i.e., 3.02+6.3mOm/l and seems hypertonic in KCS i.e., 330 to 340. This test may be more accurate for dry eye diagnosis than Schirmer’s, TBUT and corneal/ conjunctival staining [32,67].
Conjunctival biopsy and scraping
Conjunctival biopsy and scraping have been advocated to detect the histological change in dry eye. After staining the material, goblet cell densities and their morphology, and keratinization of epithelial cells, which is very specific in dry eye, can be seen microscopically [68].
Fluorophotometer
Fluorescein dilution or fluorophotometer is used to estimate tear flow, tear film thickness and variation sub-volumes of the total volume by measuring the dilatation of an initial concentration of fluorescein in tear [69]. The test is limited because of unavailability of fluorophotometer attachment with slit-lamp routinely [70].
Conjunctival impression cytology
Recently, a non-invasive technique of impression cytology, where the conjunctival impression is taken to examine cellular structure, has been used for the diagnosis of dry eye [71]. Progression of ocular surface changes such as marked decrease in goblet cell count and keratinization is monitored by collecting superficial layers and examined microscopically. the conjunctival impression cytology has been graded according to the severity of dry eye state from 0 to 5 as follows [32,54]:
Stage 0: Normal cellular structure
Stage 1: Early loss of goblet cell without keratinization Stage 2: total loss of goblet cell with slight enlargement of epithelial cells.
Stage 3: Early and mild keratinization.
Stage 4: moderate keratinization
Stage 5: Advance keratinization
Tear ferning test
The Tear Ferning Test (TFT) can be used to help diagnose the quality of tears/mucin, DES, and hyperosmolarity. A drop of tear fluid is collected from the lower eyelid and then placed onto a microscope slide and allowed to dry by evaporation. Different forms of branching crystallization patterns are observed and classified. The test diagnoses dry eyes on the basis of the ferning patterns [72].
Management
The goals of treatment are to relieve the symptoms of dry eye, improve the patient’s comfort, return the ocular surface and tear film to the normal state, and, whenever possible, prevent corneal damage [54].There is substantial variation among both eye care practitioners and countries in terms of dry eye treatment modalities. The latest 2017 International Dry Eye Workshop II report aimed to reduce these differences and emphasized the use of a stepped care algorithm [54]. The algorithm includes treatment forms ranging from artificial tear drops, the primary conventional treatment method, to the latest surgical applications. The aims of the algorithm are to restore homeostasis in the ocular surface, break the vicious cycle of inflammation, and ensure long-term ocular surface. Management of dry eye depends on its etiology.
Medical Management
Artificial tear: The basic strategy of artificial tears is to increase the amount of liquid on the ocular surface, decrease tear evaporation, and augment the lipid content or lubricity of the tears. All three are aimed at increasing tear volume or improving the quality of the tear film. Artificial tear is lubricating eye drops used to relieve dryness and irritation of the ocular surface by adding similar lubricating elements that naturally contain. Although artificial tear is used to mimic or supplement the roles of tear film and contain water, electrolytes, and certain polymers but don’t contain the biologically active components found in naturally produced tears. Moreover, artificial tears contain chemicals like carboxymethyl cellulose, polyvinyl alcohol, hyaluronic acid, hydroxypropyl cellulose and hydroxypropyl methylcellulose that are not present in naturally produced tear. It also contains preservatives in order to prolong usage and avoid bacterial contamination [73]. Sometimes these preservatives can be toxic and damage corneal epithelium so for those patients who cannot tolerate preservative artificial drop and those who use it frequently, they may use preservative-free artificial tear drop [68]. Artificial tear helps to thicken and stabilize the precorneal tear film, prolonging tear film breakup time and allowing for tear to protect the surface of the eye properly. Mild disease conditions require the application of lubricant drops four times a day while severe cases need greater frequency (10-12 times a day) of administration [74]. If a contact lens user has been diagnosed with DED he/she might be suggested to use artificial tear designed especially for people who wear contact lens [74]. Artificial tears provide palliative relief to eye irritation in patients with aqueous tear deficiency, but do not prevent the underlying inflammation or reverse conjunctival squamous metaplasia in chronic DED, therefore combination with other therapies are needed in most cases.
Autologous Serum Eye Drops
Autologous serum eye drops contain different essential tear components such as hepatocyte growth factor, epidermal growth factor, vitamin A, and fibronectin that are important for a maintaining healthy ocular surface. All these components are not available in the commercial products and use of these eye drops for treatment of KCS is controversial [75].
Anti-Inflammatory Therapy
Since inflammation is a key pathogenic factor in DED, therapeutic measures should contain anti-inflammatories. NSAID drops containing drugs such as diclofenac sodium and ketorolac reduce the inflammation associated with DED such as blepharitis, dacryoadenitis etc. In DED, to break the vicious circle of surface damage and inflammation, following anti-inflammatories are usually prescribed.
Cyclosporin A (CsA)
Cyclosporin A is effective in a number of ocular immune pathologies. Systemic administration of drug is used in treatment of local ophthalmic conditions involving cytokines, such as corneal graft rejection, autoimmune uveitis, and dry eye syndrome; however, it induces severe renal and cardiovascular complications [76]. In DED, topical application of cyclosporine A leads to increased production of tear fluid, possibly via local release of parasympathetic neurotransmitters and through an increase in goblet cell density) which consequently help in restoring epithelial damage, and reducing disease recurrences over the long term, besides its active immunosuppressant and anti-inflammatory actions. Topical CsA significantly alleviates the signs and symptoms of DE and is often prescribed for long-term use by eye care practitioners.
Topical corticosteroids
Topical corticosteroids, such as loteprednol etabonate, dexamethasone, prednisolone, and fluorometholone, are found to be effective in inflammatory conditions associated with DED and these are approved by the FDA for treating inflammatory conditions of the conjunctiva, cornea, and anterior globe [73]. They are generally recommended for short-term use as prolonged use may result in adverse effects such as ocular infection, glaucoma, and cataracts [77]. Topical steroids are also used to dampen inflammation on the ocular surface in DE, often in combination with CsA. The effect of corticosteroids on the inflammatory cascade, specifically the blockade of cyclooxygenase, production of prostanoids from arachidonic acid and stimulation of the apoptosis of lymphocytes, is well known and is likely the reason this form of therapy has been efficacious in practice [78].
Antibiotics
Antibiotics are particularly important in DED secondary to eye lid abnormalities. Ophthalmic ointments containing antibiotics such as erythromycin and bacitracin are used for treatment of meibomian gland dysfunction [79]. Tetracyclines are used in DED primarily for their anti-inflammatory effects rather than antibacterial actions [80] as they have immunomodulatory properties which have been noted to decrease ocular surface inflammation and normalize lipid production by the meibomian glands..
Surgical Management
Punctal plugs
Punctal plugs are tiny devices that are placed in puncta (two small round openings on upper eyelid and lower eyelid near medial canthus which helps drain tear to Nasolacrimal duct [81]. Punctal plugs also called lacrimal plugs or occluders are usually inserted in the puncta of the upper, lower or both eyelids. There are two variation of Punctal plugs, they are
a. Temporary/dissolving plugs which are made up of collagen material that gradually breaks down and is absorbed by body. These plugs last in eye for few days to months. These plugs are often used to keep eye moist after having refractive surgery.
b. Semi-permeable plugs which are made of longer-lasting medieval plastic such as silicone or acrylic which can remain in the eye for years and can be removed as well.
Punctal occlusion reduces drainage, preserves natural tears and prolongs the effect of lubricants. It is indicated in patients refractory to medical treatment, having a Schirmer test (with anesthesia) result of less than 5mm at 5min, and showing the evidence of ocular surface dye staining [82]. Permanent punctal occlusion may be achieved surgically using cauterization.
Salivary gland procedures
Since 1951, surgical procedures involving salivary glands for the management of DE have been explored. Filatov and Chevalijev [83] have described the parotid duct transfer to the conjunctival fornix, while Murube [84] has described the transfer of the submandibular salivary gland to the temporal region and implant of the Wharton duct into the upper fornix for DED treatment. Studies have also reported the use of a graft of labial mucosa and minor salivary glands to treat severe dry eye [85].
Subcutaneous abdominal artificial tear pump-reservoir
The artificial tear pump-reservoir was suggested by Murube for the treatment of severe dry eye [86] It was implanted into a subcutaneous pocket of the anterolateral abdominal wall and the silicon tube catheter is passed via chest, neck and face to the upper conjunctival fornix.
Supplementary Therapy
Vitamin A
Vitamin A plays vital role in vision. Vitamin A is an essential nutrient present naturally in tear film of healthy eyes and plays an important role in production of the mucin layer, the innermost lubricating layer of tear film. Vitamin A deficiency leads to loss of mucin layer and goblet cell atrophy. It protects the eyes from free radicals, toxins, allergens, and inflammation. Topical retinoic acid therapy in conjunction with systemic administration of vitamin A has been investigated to treat xerophthalmia and Bitot’s spots [87].
Essential fatty acids (EFAs)
Oral supplementation with essential fatty acids (EFAs) is suggested nowadays as a supplementary treatment for DED. EFAs are the precursors of eicosanoids (prostaglandins, prostacyclins, thromboxanes, and leukotrienes) that modulate immune responses; while omega-3 FAs are generally classified as anti-inflammatory, omega-6 FAs are considered proinflammatory [88] It appears that omega-3 fatty acids can improve the eye’s oil film that’s produced by small glands on the edge of the eyelid, called the meibomian glands [89].
Nerve growth factor (NGF)
NGF has been observed to increase ocular surface sensitivity, inhibit inflammatory reactions and regulate tear film production [90] Thus, NGF seems to a play a pivotal role in the pathophysiology of DE and may be a promising therapeutic option [91].Exogenous NGF administration may be beneficial in recovering ocular surface damage due to chronic hyperosmolarity [91].
Hormonal therapy
Receptors for androgens, estrogens, progesterone and prolactin have been identified in several ocular tissues, including the lacrimal gland and meibomian glands [92-94]. Studies have demonstrated that adequate androgen, prolactin and estrogen levels are essential for normal lacrimal gland function and structural organization [95- 97]. Administration of topically applied androgen and estrogen steroid hormones for 3-4 months has also been found to show clinical improvement in the form of increased tear production TBUT and lipid layer thickness with corresponding symptomatic relief [98,99].
Acupuncture
The use of acupuncture as a treatment for eye disease is based on the claims that acupuncture modulates autonomic nervous system and immune system [100], which in turn might regulate lacrimal gland function and thus help to increase tar production in DED patients.
Lifestyle and dietary approaches
Lifestyle approaches to the management of dry eye include ensuring adequate fluid intake, moderating alcohol use, using humidifiers or protective eyewear, and when possible, avoiding air conditioning and forced-air heating. Sleep deprivation can trigger dry eye symptoms [101] so adequate sleep is also important. Certain foods, such as fish and flaxseed, contain n-3 and n-6 fatty acids which aids in tear production.
Prognosis of DED
If we will be unable to control DED or do not treat it properly then it will be further progress as allergic conjunctivitis, corneal ulcer, induce headache and eye ache, inability to wear contact lens etc. [7,49].
Current Challenges and Future Aspects
Since dry eye disease is ubiquitous and under diagnosis ocular morbidities. The prevalence of DED is increasing gradually day by day, in other hand the diagnosis of dry eye is challenging due to extensive variety of signs and symptoms and the ambiguity in the etiology and pathophysiology of the disease. Several factors contribute to making diagnosis difficult and warrant further attention: the invasiveness and low degree of standardization of most conventional tests (Schirmer, TBUT and ocular surface staining), the still incomplete knowledge about the pathophysiology underlying the phenomena measured by some of these tests (e.g. BUT) and the overlapping of dry eye symptoms with those of other conditions, such as conjunctivochalasis (which can easily induce an unstable tear film) or delayed tear clearance (which is a frequent cause of ocular irritation). Because of the variety of causes and several factors involved in tear film instability, practitioners should incorporate more than one tests into a pre-examination routine. There is substantial variation among both eye care practitioners and countries in terms of dry eye treatment modalities so certain strategy should be made in order to reduce these differences and emphasize the use of a stepped care algorithm. Furthermore, research should be conducted in order to know proper etiology of dry eye.
Summary
Differentiation of severe dry eye versus allergic conjunctivitis is important in a clinical setting. New technology is available to facilitate this process, which produces results within the span of a single patient visit. Thus, a practitioner can be confident of the diagnosis and proper treatment protocol on the first visit and while the patient is still in the chair. The reimbursable tests can be performed by a clinician and can help to establish the practice as a leader in dry eye resolution.
References
- (2007) The definition and classification of dry eye disease: Report of the definition and classification subcommittee of the international Dry Eye Workshop. Ocular Surface 5(2): 75-92.
- Delaleu N, Jonsson R, Koller MM (2005) Sjögren's syndrome. Eur J Oral Sci 113(2): 101-113.
- Stevenson W, Chauhan SK, Dana R (2012) Dry eye disease: An immune-mediated ocular surface disorder. Arch Ophthalmol 130(1): 90-100.
- Denoyer A, Rabut G, Baudouin C (2012) Tear film aberration dynamics and vision-related quality of life in patients with dry eye disease. Ophthalmology 119(9): 1811-1818.
- Stapleton F, Alves M, Bunya VY (2017) TFOS DEWS II Epidemiology Report. Ocul Surface 15(3): 334-365.
- Farrand KF, Fridman M, Stillman IO, Schaumberg DA (2017) Prevalence of diagnosed dry eye disease in the united states among adults aged 18 years and older. Am J Ophthalmol 182: 90-98.
- Sharma B (2011) Dry eye: Demography and attributable. Postgraduate Medical Journal of NAMS 11(1): 16-22.
- Caffery BE, Richter D, Simpson T, Fonn D, Doughty M, et al. (1998) Candees. The Canadian dry eye epidemiology study. Adv Exp Med Biol 438: 805-806.
- Van Landingham SW, West SK, Akpek EK, Muñoz B, Ramulu PY (2014) Impact of dry eye on reading in a population-based sample of the elderly: The salisbury eye evaluation. Br J Ophthalmol 98(5): 639-644.
- Shimmura S, Shimazaki J, Tsubota K (1999) Results of a population based questionnaire on the symptoms and lifestyles associated with dry eye. Cornea 18(4): 408-411.
- Moss SE, Klein R, Klein BE (2000) Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 118(9): 1264-1268.
- Lee AJ, Lee J, Saw SM, Gazzard G, Koh D, et al. (2002) Prevalence and risk factors associated with dry eye symptoms: A population based study in Indonesia. Br J Ophthalmol 86(12): 1347-1351.
- Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, et al. (2003) Prevalence and association of dry eye in an older population: The blue mountain eye study. Clin Experiment Ophthalmol 31(3): 229-232.
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR (2003) Prevalence of dry eye syndrome among US women. Am J Ophthalmol 136(2): 318-326.
- Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, et al. (2003) Prevalence of dry eye among an elderly Chinese population in Taiwan: the shihpai eye study. Ophthalmology 110(6): 1096-1101.
- Sahai A, Malik P (2005) Dry Eye: Prevalence and attributable risk factors in a hospital based population. Ind J Ophthalmol 53(2): 87-91.
- Lekhanont K, Rojanaporn D, Chuck RS, Vongthongsri A (2006) Prevalence of dry eye in Bangkok, Thailand. Cornea 25(10): 1162-1167.
- Gupta N, Prasad I, Himashree G, D Souza P (2008) Prevalence of dry eye at high altitude: A case controlled comparative study. High Alt Med Biol 9(4): 327-334.
- Jie Y, Xu L, Wu YY, Jonas JB (2009) Prevalence of dry eye among adult Chinese in the Beijing Eye Study 23(3): 688-693.
- Bukhari A, Ajlan R, Alsaggaf H (2009) Prevalence of dry eye in the normal population in Jeddah, Saudi Arabia. Orbit 28(6): 392-397.
- Gupta N, Prasad I, Jain R, D’Souza P (2010) Estimating the prevalence of dry eye among Indian patients attending a tertiary ophthalmology clinic. Ann Trop Med Parasitol 104(3): 247-255.
- Han SB, Hyon JY, Woo SJ, Lee JJ, Kim TH, et al. (2011) Prevalence of dry eye disease in an elderly Korean population. Arch Ophthalmol 129(5): 633-638.
- Viso E, Gude F, Rodríguez-Ares MT (2011) The association of meibomian gland dysfunction and other common ocular diseases with dry eye: A population based study in Spain. Cornea 30(1): 1-6.
- Basak SK, Pal PP, Basak S, Bandyopadhyay A, Choudhury S, et al. (2012) Prevalence of dry eye diseases in hospital-based population in West Bengal, Eastern India. J Indian Med Assoc 110: 789-794.
- Uchino M, Yokoi N, Uchino Y, Dogru M, Kawashima M, et al. (2013) Prevalence of dry eye disease and its risk factors in visual display terminal users: The Osaka study. Am J Ophthalmol 156(4): 759-766.
- Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, et al. (2014) Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am J Ophthalmol 157(4): 799-806.
- Shah S, Jani H (2015) Prevalence and associated factors of dry eye: Our experience in patients above 40 years of age at a tertiary care center. Oman J Ophthalmol 8(3): 151-156.
- Inomata T, Shiang T, Iwagami M, Sakemi F, Fujimoto K, et al. (2018) Changes in distribution of dry eye disease by the new 2016 diagnostic criteria from the Asia dry eye society. Sci Rep 8: 1-7.
- Dana R, Bradley JL, Guerin A, Pivneva I, Stillman IO, et al. (2019) Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age united states health care system. Am J Ophthalmol 202: 47-54,
- Shanti Y, Shehada R, Bakkar MM, Qaddumi J (2020) Prevalence and associated risk factors of dry eye disease in 16 northern West bank towns in Palestine: A cross-sectional study. BMC Ophthalmol 20(1): 26.
- Holland EH, Mannis MJ, Lee WB (2013) Ocular surface of disease: Cornea, conjunctiva and tear film, Saunders Publishers, USA.
- Khurana AK (2006) Anatomy and physiology of eye, CBS Publishers, India.
- Peters E, Colby K (2006) The tear film. Tasman W, Jaeger EA (Eds.), Physiology of the Eye and Visual System. Duane's foundations of clinical ophthalmology, Lippincott Williams & Wilkins, Philadelphia, USA, 2.
- Johnson ME, Murphy PJ (2004) Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res 23(4): 449-474.
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, et al. (1998) A unified theory of the role of the ocular surface in dry eye. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2, pp. 643-651.
- (2007) Report of epidemiology sub- committee on international dry eye workshop. Ocul Sur 5(2): 93-107.
- Ang RT, Dartt DA, Tsubota k (2001) Dry eye after refractive surgery. Curr Opin Ophthalmol 12(4): 318-322.
- WHO (1976) Vitamin A defficency and xeropthalmia.
- Xu L, Zhang W, Yu XZ, Suo T, Qun XF (2016) Smoking and the risk of dry eye: A meta-analysis. Int J Ophthalmol 9(10): 1480-1486.
- Kaiti R, Shah P, Bogati B, Shyangbo R, Hamal B, et al. (2020) Computer vision syndrome: Is it being diagnosed and managed properly? Acta Scientific Ophthalmology 3(7): 13-20.
- Fraunfelder FT, Fraunfelder FW, Chambers WA (2008) Clinical ocular toxicology, Saunders Publishers, USA.
- Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, et al. (2010) An Objective Approach to Dry Eye Disease Severity. Invest Ophthalmol Vis Sci 51(12): 6125-6130.
- Uchiyama E, Aronowicz JD, Igor AB, McCulley JP (2007) Increased evaporative rates in laboratory testing conditions simulating airplane cabin relative humidity: An important factor for dry eye syndrome. Eye & Contact Lens 33(4): 174-176.
- Lemp MA (1995) Report of the National eye institute/industry workshop on clinical trials in dry eyes. CLAO J 21(4): 221-232.
- Liu Z (2004) The preliminary recommendations on the name and classification of dry eye (in Chinese). Chin J Eye Otolaryngol 3: 4-5.
- Murube J, Németh J, Höh H, Hekimhan PK, Winter JH (2005) The triple classification of dry eye for practical clinical use. Eur J Ophthalmol 15(6): 660-667.
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, et al. (2017) TFOS DEWS II definition and classification report. Ocul Surf 15(3): 276-283.
- Tsubota K, Yokoi N, Watanabe H, Dogru M, Kojima T, et al. (2019) A New perspective on dry eye classification: Proposal by the Asia dry eye society. Eye Contact Lens 46 Suppl 1(1): S2-S13.
- (2008) Dry eye. Mayo Foundation for Medical Education and Research.
- Meadows M (2005) Dealing with dry eye. FDA Consum 39(3): 8-9.
- Dry Eye Syndrome. Wikipedia.
- Uchino M, Schaumberg DA (2013) Dry eye disease: Impact on quality of life and vision. Curr Ophthalmol Rep 1(2): 51-57.
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, et al. (2001) Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci 42(10): 2283-2292.
- (2007) Methodologies to diagnose and monitor dry eye disease: Report of the diagnostic methodology subcommittee of the international dry eye workshop. Ocular Surface 5(2): 108-152.
- Clinch TE, Benedetto DA, Felberg NT, Laibson PR (1983) Schirmer's test. A closer look. Arch Ophthalmol 101(9): 383-1386.
- Senchyma M, Wax MB (2008) Quantitative assessment of tear production: A review of methods and utility in dry eye drug discovery. J Ocul Biol Dis Infor 1(1): 1-6.
- Cho P, Yap M (1993) Schirmer test. I. A review. Optom Vis Sci 70(2): 152-156.
- Cho P, Yap M (1993) Schirmer test. II. A clinical study of its repeatability. Optom Vis Sci 70(2): 157-159.
- Tsubota K (2018) Short tear film breakup time-type dry eye. Invest Ophthalmol Vis Sci 59(14): 64-70.
- Kallarackal GU, Ansari EA, Amos N, Martin JC, Lane C, et al. (2002) A comparative study to assess the clinical use of fluorescein meniscus time (FMT) with tear break up time (TBUT) and Schirmer’s tests (St) in the diagnosis of dry eyes. Eye (Lond) 16(5): 594-600.
- Mengher LS, Bron AJ, Tonge SR, Gilbert DJ (2018) A non-invasive instrument for clinical assessment of the pre-corneal tear Film stability. Curr Eye Res 4(1): 1-7.
- Mainstone JC, Bruce AS, Golding TR (1996) Tear meniscus measurement in the diagnosis of dry eye. Curr Eye Res 15(6): 653-661.
- Lim DK, Berry M (2003) Still confused about rose Bengal? Invest Ophthalmol Vis Sci 29(4-5): 311-317.
- (1998) Comparison of rose Bengal and lissamine green conjunctival staining in dry eye patients. American Academy of Optometry.
- Anshel J (2020) Lactoferrin levels can diagnose dry eye disease. Optometry Times 12(6): 11.
- Avisar R, Menaché R, Shaked P, Rubinstein J, Machtey I, et al. (1979) Lysozyme content of tears in patients with sjögren's syndrome and rheumatoid arthritis. Am J Ophthalmol 87(2): 148-151.
- Potvin R, Makari S, Rapuano CJ (2015) Tear film osmolarity and dry eye disease: A review of the literature. Clin Ophthalmol 9: 2039-2047.
- Murube J, Rivas L (2003) Impression cytology on conjunctiva and cornea in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol 13(2): 115-127.
- Fahim MM, Haji S, Koonapareddy CV, Fan VC, Asbell PA (2006) Fluorophotometry as a diagnostic tool for the evaluation of dry eye disease. BMC Ophthalmol 6: 1-7.
- Wang HF, Fukuda M, Shimomura Y (2009) Diagnosis of dry eye. Seminars in Ophthalmology 20(2): 53-62.
- Rolando M, Terragna F, Giordano G, Calabria G (1990) Conjunctival surface damage distribution in keratoconjunctivitis sicca. An impression cytology study. Ophthalmologica 200(4): 170-176.
- Vaikoussis E, Georgiou P, Nomicarios D (1994) Tear mucus ferning in patients with Sjogren's syndrome. Doc Ophthalmol 87(2): 145-151.
- Gupta SK (2014) Textbook on clinical ocular pharmacology and therapeutics. In: Agarwal R, Srivastava S, Gupta SK (Eds.), Jaypee Brothers Medical Publishers, India.
- Moshirfar M, Pierson K, Hanamaikai K, Caban LS, Muthappan V et al. (2014) Artificial tears potpourri: A literature review. Clin Ophthalmol 8: 419-1433.
- Geerling G, Maclennan S, Hartwig D (2004) Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol 88(11): 1467-1474.
- Schein OD, Muñoz B, Tielsch JM, Roche KB, West SK (1997) An epidemiologic study of medication uses and symptoms of dry eye. Investigative Ophthalmology and Visual Science 38(1): 213.
- Steroid and eye. UK Ultra Vision Strategy, UK.
- Aksoy MO, Li X, Borenstein M, Yi Y, Kelsen SG (1999) Effects of topical corticosteroids on inflammatory mediator-induced eicosanoid release by human airway epithelial cells. J Allergy Clin Immunol 103(6): 1081-1091.
- Perry HD, Carnevale SD, Donnenfeld ED, Solomon R, Biser SA, et al. (2006) Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea 25(2): 171-175.
- Gilbard JP (2004) Ophthalmic solution with tetracycline for topical treatment of dry eye disease. Advanced Vision Research EP 1105139 B1.
- Qiu W, Liu Z, Zhang Z, Ao M, Li X, et al. (2012) Punctal plugs versus artificial tears for treating dry eye: A comparative observation of their effects on contrast sensitivity. J Ocul Biol Dis Infor 5(1): 19-24.
- Baxter SA, Laibson PR (2004) Punctal plugs in the management of dry eyes. Ocul Surf 2(4): 255-265.
- Tavares FP, Fernandes RS, Bernardes TF, Bonfioli AA, Soares EJC (2010) Dry eye disease. Semin Ophthalmol 25(3): 84-93.
- Geerling G, Sieg P, Bastian GO, Laqua H (1998) Transplantation of the autologous submandibular gland for most severe cases of keratoconjunctivitis sicca. Ophthalmology 105(2): 327-335.
- Guerrissi JO, Belmonte J (2004) Surgical treatment of dry eye syndrome: Conjunctival graft of the minor salivary gland. J Craniofac Surg 15(1): 6-10.
- Murube J, Murube E, Zhuo LC, Rivas L (2003) Subcutaneous abdominal artificial tears pump-reservoir for severe dry eye. Orbit 22(1): 29-40.
- Gilbert C (2013) The eye signs of vitamin A deficiency. Community Eye Health 26(84): 66-67.
- Rosenberg ES, Asbell PA (2010) Essential fatty acids in the treatment of dry eye. Ocul Surf 8(1): 18-28.
- Al Mahmood AM, Al-Swailem SA (2015) Essential fatty acids in the treatment of dry eye syndrome: A myth or reality? Saudi J Ophthalmol 28(3): 195-197.
- Lee HK, Lee KS, Kim HC, Lee SH, Kim EK (2005) Nerve growth factor concentration and implications in photorefractive keratectomy vs laser in situ keratomileusis. Am J Ophthalmol 139(6): 965-971.
- Lambiase A, Mantelli F, Sacchetti M, Rossi S, Aloe L, et al. (2011) Clinical applications of NGF in ocular diseases. Arch Ital Biol 149(2): 283-292.
- Coassin M, Lambiase A, Costa N, De Gregoria A, Srulletta R, et al. (2005) Efficacy of topical nerve growth factor treatment in dogs affected by dry eye. Graefes Arch Clin Exp Ophthalmol 243(2): 151-155.
- Khandelwal P, Liu S, Sullivan DA (2018) Androgen regulation of gene expression in human meibomian gland and conjunctival epithelial cells. Mol Vis 18: 1055-1067.
- Sullivan DA, Rocha EM, Ullman MD, Krenzer KL, Gao J, et al. (1998) Androgen regulation of the meibomian gland. Adv Exp Med Biol 438: 327-331.
- Sullivan DA (2013) Tearful relationships? sex, hormones, the lacrimal gland, and aqueous-deficient dry eye. Ocul Surf 2(2): 92-123.
- Greenblatt RB, Colle ML, Mahesh VB. Ovarian and adrenal steroid production in the postmenopausal woman. Obstet Gynecol 47(4): 383-387.
- Gagliano C, Caruso S, Napolitano G, Malaguarnera G, Cicinelli MV, et al. (2014) Low levels of 17-beta-oestradiol, oestrone and testosterone correlate with severe evaporative dysfunctional tear syndrome in postmenopausal women: A case-control study. Br J Ophthalmol 98(3): 371-376.
- Worda C, Nepp J, Huber JC, Sator MO (2001) Treatment of keratoconjunctivitis sicca with topical androgen. Maturitas 37(3): 209-212.
- Sator MO, Joura EA, Golaszewski T, Gruber D, Frigo P, et al. (1998) Treatment of menopausal keratoconjunctivitis sicca with topical oestradiol. Br J Obstet Gynaecol 105(1): 100-102.
- Kavoussi B, Ross BE (2007) The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther 6(3): 251-257.
- Lee YB, Koh JW, Hyon JY, Wee WR, Kim JJ, et al. (2014) Sleep deprivation reduces tear secretion and impairs the tear film. Invest Ophthalmol Vis Sci 55(6): 3525-3531.
© 2020 Raju Kaiti. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






