- Submissions

Full Text
Gastroenterology Medicine & Research
The Potential Role of Circulating Inflammatory Cells in Predicting the Safety, Efficacy and Immune-related Adverse Events of Immune Check-point Inhibitors: A Narrative Review
Tibera K Rugambwa1,2*, Omar Abdihamid2,3,4, Miriam Kessy5 and Alphonce MK Nyalali6,7,8
1Department of Internal Medicine, Mbeya Zonal Referral Hospital and Mbeya College of Health and Allied Sciences, University of Dar-es-salaam, Mbeya, Tanzania
2Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan, China
3HCGCCK Cancer Centre, Nairobi, Kenya
4Garissa Cancer Centre, Garissa County, Kenya
5Department of Pediatrics, Xiangya Hospital, Central South University, Changsha, Hunan, China
6Department of Neurosurgery, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan 250117, China.
7Department of Neurosurgery, Qilu Hospital of Shandong University, Cheeloo College of Medicine, Shandong University, Jinan 250012, China
8Department of Surgery, Songwe Regional Referral Hospital, Songwe 23, Mbeya, Tanzania
*Corresponding author:Tibera K Rugambwa, Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan, China
Submission:March 09, 2023;Published: March 30, 2023

ISSN 2637-7632Volume7 Issue3
Abstract
Immune checkpoint blockade has influenced the course of cancer treatment with improved Overall Survival (OS) and durable responses in many malignancies. Despite these responses, their mechanism of action has brought different set of adverse events that are severe and life-threatening in a subset of patients. Moreover, not all patients have shown to benefit from this treatment although their tumors express U.S. Federal and Drug Administration (FDA) approved biomarkers i.e programmed cell Death Protein 1 (PD-1) and its ligand (PD-L1), Microsatellite Instability (MSI) and Tumor Mutation Burden (TMB). Limitations of these markers has stimulated further research to identify and incorporate other markers that could serve as an adjunct to the current approved ones. Circulating inflammatory cells are among biomarkers that have shown to have a prognostic and predictive value in determining treatment response to Immune Checkpoint Inhibitors (ICI) and risk of Immune-Related Adverse Events (irAEs). This review aims to provide more details on the key inflammatory cells that play a pivotal role in cancer progression and inhibition and how their interaction in the tumor micro-environment (TME) affects response to immune checkpoint blockade and predicting irAEs. Moreover examples of prognostic models that have incorporated these cells will be highlighted.
Keywords:Immune checkpoint inhibitors; Circulating inflammatory cells; Immune-related adverse events; Biomarkers; Efficacy; Safety
Introduction
Inflammation plays a dual role of inhibition and promotion with regards to cancer growth and metabolism. Hence tumor promoting inflammation is identified as one of the hallmarks of cancer [1]. In addition, inflammation as an immunological response also contributes to other core hallmarks of cancer such as angiogenesis, invasion and metastasis [1]. Inflammatory peripheral blood cells that are mainly involved in promotion and inhibition of cancer are lymphocytes, neutrophils, eosinophils and platelets. Lymphocytes and neutrophils which make up the bulk of immune system constitute the greatest percentage of leucocytes. Lymphocytes function in adaptive response to eliminate cancer cells while neutrophils function in innate immunity and release signals during adaptive immune response that inhibit activity of cytotoxic T-lymphocytes [2]. Both leukocytes and neutrophils also secrete cytokines, chemokines like Tumor Growth Factor-Beta (TGF-β), Vascular Endothelial Growth Factor (VEGF), IL-6, IL-8, IL-12 and matrix metalloproteinases (MMPs) that promote angiogenesis hence cancer growth [2]. Platelets contribute to tumorigenesis by secreting Platelet Derived Growth Factor (PDGF) and VEGF which mediate extravasation and migration of cancer cells. Eosinophils are mainly involved in immune response against multicellular and macroscopic parasites and in allergic reactions. Also, they regulate other subsets of immune cells in Tumor Microenvironment (TME) depending on different factors. Induced cytotoxicity towards cancer cells result to antitumor or protumor effects [3]. Immune Checkpoint Inhibitors (ICIs) which form part of immunotherapy target Programmed Death-1 (PD-1), Programmed Death Ligand 1 (PDL-1) and Cytotoxic T-Lymphocyte Associated Antigen 4 (CTLA- 4) which are innate negative T-cell regulators. Inhibition of these molecules activate immune system hence augment ability of immune cells to recognize and destroy tumor cells [4]. However, activated immune system cause off-target and bystander effects to the normal tissues resulting in Immune Related Adverse Events (irAEs) akin to autoimmune diseases such as colitis, pneumonitis, thyroiditis, hepatitis among others which are signature irAEs of ICIs.
Hypotheses that explain some of these effects include disruption of central and peripheral tolerance from blocking immune checkpoints, cross presentation of shared antigens between cancer cells and normal tissue, epitope spreading caused by treatment induced inflammation, genetic predisposition to autoimmune diseases, organ-specific expression of immune checkpoints. Lastly gut microbiota is also identified to contribute to irAEs especially colitis [5]. As more ICI agents are being developed and approved for various tumors in first line or metastatic settings and in both neo adjuvant and adjuvant settings, it is important to identify biomarkers that can predict efficacy of these agents as well as risk factors for irAEs [2]. Currently PDL-1 levels, Microsatellite Instability status (MSI) and Tumor Mutation Burden (TMB) are FDA approved biomarkers to determine patients more likely to benefit from ICIs [6]. However not all patients whose tumors express high PD-L1 levels or MSI-H or with high TMB respond to ICIs. There is a significant percentage that progress rapidly while on ICIs the so-called hyper-progression [7,8]; others progress temporarily (pseudoprogression) defined as transient radiological increase in width and diameter of the primary lesion resulting from over stimulation of immune system [8]; some patients confer little or no response from ICIs and might show early signs of resistance [9,10]. In addition, identifying biomarkers that could predict occurrence of irAEs is an area of ongoing research. Lymphocytes, neutrophils, eosinophils and platelets which are the main inflammatory cells, are useful and independent predictors of survival and benefit from ICIs and predicting the risk of irAEs [11]. High Neutrophil to Lymphocyte Ratio (NLR) and high Platelet to Lymphocyte Ratio (PLR) have been associated with worse Overall Survival (OS) in numerous tumors [2]. In a univariate analysis, changes in serum peripheral blood cell count during immunotherapy were noted to relate with the development of irAEs especially colitis and pneumonitis [12]. Similarly, reduction in peripheral T-lymphocyte count has a negative impact on response to ICIs as ICIs depend on inhibitory signal of T-lymphocytes [2]. Several studies have investigated the relationship between inflammatory cells and immunotherapy. One review highlighted different blood inflammatory markers and their ratios in cancer immunotherapy and how they were associated with Progression Free Survival (PFS) and OS in different solid tumors treated with ICIs [2].
Yang F et al. [13] examined emerging data for novel biomarkers including tumor intrinsic based markers, TME-based markers and patient-based markers that may have a predictive value for optimizing treatment benefit from ICIs [13]. Despite such analysis, there is a need to show how blood cells that make part of TME could be combined with other markers to make a prognostic and predictive model that could be an adjunct to already FDA approved markers in determining the benefit and the harms of ICIs. This review aims to provide details on the key inflammatory cells that play a pivotal role in cancer progression and inhibition and how their interaction in TME affects response to immune checkpoint blockade and predicting irAEs. Also examples of already existing prognostic and predictive models that have incorporated these cells will be highlighted.
Inflammatory Blood Cells in Routine Complete Blood Count
Lymphocytes
Lymphocytes contribute up to 40% of leukocytes in a sample of healthy adult blood and are the functional unit in adaptive immune response. Lymphocytes are divided into T-lymphocytes, B-lymphocytes and Natural Killer (NK) cells. However T Cell Lymphocytes (TCLs) play a bigger role in fighting cancer cells as they are the major actors in controlling tumor progression in many human cancers. Peripheral lymphocytosis reflects a state of T cell activation [14]. Whereas reduction in lymphocytes affect antitumor response [2]. The relationship of T-cell lymphocytes with other inflammatory cells and their effect in cancer is demonstrated in Figure 1.
Figure 1:Demonstration of the interaction between T cell lymphocytes and other inflammatory cells in relation to
cancer suppression or progression.
Abbreviations: ADP: Adenosine Diphosphate; ARG1: Arginase 1; CCL5: Chemokine Motif Ligand 5; CTCs:
Circulating Tumor cells; CXCL9: Chemokine Ligand 9; CXCL10: Chemokine Ligand 10; FOXP3: Forkhead Box
Protein P3; GM-CSF: Granulocyte Macrophage Colony Stimulating Factor; iNOS: Inducible Nitric oxide synthase;
IRS1:Insulin Receptor Substrate 1; MQs: Macrophages; MMPs: Matrix Metalloproteinases; MET: Mesenchymal
Epithelial Transition Factor; NK cells: Natural Killer cells; PD-1: Programmed Death 1; PI3K: Phosphoinositide
3-Kinase; PROK2: Prokineticin Receptor 2; ROS: Reactive Oxygen Species; TME: Tumor Microenvironment; TF-IRF-
5:I Interferon Regulatory Factor 5; TF: Thrombin Factor; TXA2:Thromboxane A2; TGF-beta: Tumor Growth Factor
beta; VEGF-A: Vascular Endothelial Growth Factor A.
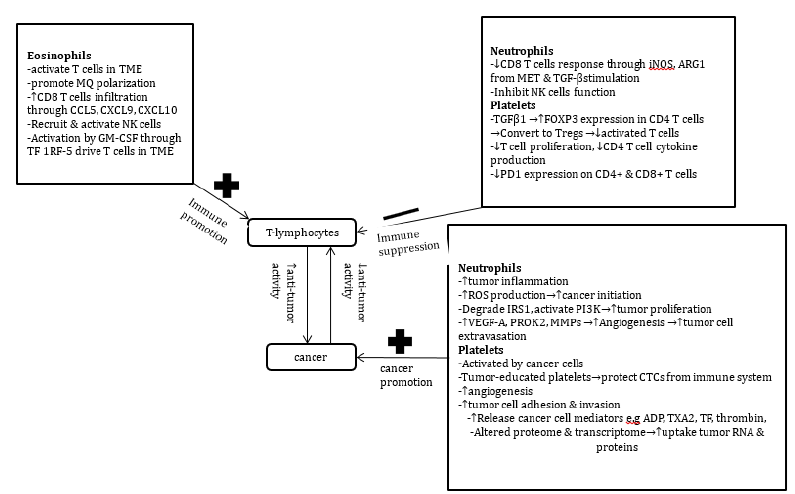
Role of T-cell lymphocytes in tumor inhibition
The important subsets of TCLs that are T helper CD4, Cytotoxic CD8 and Tregs are extensively studied in immuno-oncology. A high density of tumor-infiltrating T lymphocytes (TILs) such as CD8 T cells in the TME correlate with better prognosis in terms of Disease- Free Survival (DFS) and Overall Survival (OS) [15]. Meanwhile accumulation of Tregs, Myeloid-Derived Suppressor Cells (MDSCs) and type-2 macrophages create an immunosuppressive microenvironment hence favour tumor growth and progression. CD4 T cell lymphopenia, which occurs more in immunocompromised HIV patients and organ transplant patients predispose to increased incidence of viral related tumors like Kaposi sarcoma. Similarly, CD4 T cell lymphopenia is noted in other solid tumors like Hepatocellular Carcinoma (HCC), pancreatic cancer, Head and Neck Cancers (HNSCC), melanomas and hematological malignancies in advanced and metastatic disease [15]. The Tumor Microenvironment (TME) contains immune cells which can exhibit both pro-tumor and antitumor activity therefore Tumor Infiltrating Lymphocytes (TILs) that result from circulating lymphocytes form part of tumor stroma. Their presence signifies immune activation and has been associated with improved clinical outcomes [16]. Characteristically, TILs as reviewed in tumor samples have different peri-tumor and intratumor which serve in controlling tumor growth and metastasis [17].
T-cells as a predictor of treatment efficacy and irAEs
Although high lymphocyte count is associated with better clinical outcomes, a hyper-activated immune system increases the risk of developing irAEs. A retrospective study indicated that leukocytosis and lymphopenia fluctuation as a potential signal of irAEs especially colitis and pneumonitis. Conversely, lymphocytosis was related with the development of grade 3 or 4 irAEs [12]. Another study showed a correlation between lymphocyte count and risk of irAEs in both univariate and multivariate analysis [18]. Likewise, a study by Rilan et al. [19] showed that a lower relative lymphocyte count (cut off=28.5%) was among independent predictors of irAEs. These findings could be explained by the hypothesis that redistribution of systemic blood cells might lead to infiltration of lymphocytes in involved organs leading to reduced circulating lymphocytes [19]. A routine blood work-up in 167 adult solid tumor patients treated with nivolumab or pembrolizumab at a single institution showed that patients with an absolute lymphocyte count (ALC >2000) at baseline had an increased risk of irAEs (OR 1.996, p<0.05). Therefore, patients with persistent lymphopenia from baseline had a shorter time to disease progression compared to those who recovered (HR 2.01, p<0.05) [20]. Such findings could be attributed by the fact that a state of prolonged low lymphocyte count reflect dysfunctional T cells with limited capacity to mount a significant anti-tumor response in immune checkpoint blockade. Immune checkpoint inhibitors function by inhibiting the regulatory part of immune system. This inhibition might lead to abnormal activation of silent autoimmune T-lymphocytes resulting to irAEs [21]. Previous history of Acquired Immunodeficiency Disease (AID) or presence of active disease is proven risk factor for developing irAEs [22]. Therefore, cells in the peripheral circulation could be used to predict tumor response and probably predict occurrence of irAEs [19]
Neutrophils
Neutrophils constitute up to 70% of white blood cells, making them the most abundant circulating blood cells. Stimulation by Granulocyte-macrophage Colony Stimulating Factor (GM-CSF) and Interleukin-3 (IL-3) makes multipotent stem cells differentiate into myeloid stem cells. Myeloid stem cells give rise to granulocyte/ monocyte progenitor. With interaction of other stimulatory chemokines and cytokines, myeloid stem cells further differentiate into neutrophils and monocytes. Morphologically, neutrophils have multilobulated nuclei and small pink cytoplasmic granules. Neutrophils function by phagocytizing extracellular pathogens and release digesting enzymes that kill pathogens.
Role in cancer promotion
Neutrophil maturation period in cancer is approximately 17 hours [23] and their role in pro-tumor and anti-tumor activity is well established [2]. Furthermore, markers of Low-Density Neutrophils (LDNs) also previously described as Polymorphonuclear- Myelodysplastic Stem Cells (PMN-MDSCs) are noted to increase in cancer patients. LDNs are associated with pro-tumor characteristics compared to mature High-Density Neutrophils (HDNs) [24].
Neutrophils also exhibit suppressive function in cytotoxic T lymphocytes [25]. Activation by TGFβ induces neutrophils to Release Nitric Oxide Synthase (iNOS) and Arginase 1 (ARG1) which then inhibit CD8 TCLs [23]. Other Myeloid Derived Cells (MDSCs) suppress function of CD8+ TCLs through the same mechanism [26]. Increased neutrophils have been associated with an increase of regulatory T cells (Tregs) [14]. Additionally, neutrophils are noted to interact with Circulating Tumor Cells (CTCs) at tumor site, as CTC-neutrophil clusters are higher in tumor draining vessels. Further release of VEGF, MMPs from neutrophils promote tumor growth and metastasis [27].
Predicting survival, treatment response and irAEs
In line with the understanding of the interaction between neutrophils and lymphocytes, multiple studies have evaluated this relationship and its role in predicting and prognosticating treatment efficacy and survival. Neutrophil-Lymphocyte Ratio (NLR) and its counterpart Derived NLR (dNLR) are extensively studied. NLR is one biomarker and is defined as the ratio between absolute number of neutrophils and lymphocytes. Derived Neutrophil-To- Lymphocyte Ratio (dNLR) is another novel potential biomarker for systemic inflammation, which is calculated by absolute value of neutrophils and value of leucocyte count. dNLR maybe more linked with survival and outcomes because it includes monocytes and other granulocytes. Immature or poorly differentiated neutrophils can be released in a proinflammatory environment, which increases neutrophil generation rapidly. dNLR seems to reflect this negative inflammation more comprehensively [28]. Increased number of neutrophils or persistent higher NLR is associated with reduced benefit and response to ICIs and poor OS [29]. Likewise, the number of neutrophils and lymphocytes has been shown to predict irAEs [25]. In this study, a low pretreatment NLR was associated with higher risk of irAEs. Low NLR reflects lower peripheral neutrophil count with higher number of lymphocytes. Lymphocytosis indicates activated immune system hence increasing possibility of attacking self-antigens causing irAEs. A similar study by Lee et al showed that a NLR < 3 was a significant predictor of developing irAEs. These findings seem to be consistent with the hypothesis that NLR is a measure of PMN-MDSCs suppressing non-specific inflammation and autoimmune response mediated by TCLs due to the effect of ICIs [26].
In a case-control study of 110 participants with the secondary aim of testing the association between baseline and on-treatment ALC and NLR with subsequent Major Adverse Cardiac Events (MACEs), showed that decrease in ALC and greater increase in NLR were associated with occurrence of MACEs and had a predictive value [30]. Furthermore, a study on gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events, noted an elevation of on-treatment expression of neutrophil activation markers CD177 and CEACAM1 [31]. A similar study by Fujisawa et al. [12] noted elevated levels of White Blood Cells (WBC), decreased relative Lymphocyte Count (RLC) and increased Relative Neutrophil Count (RNC) had a significant correlation with development of lung and gastrointestinal irAEs [12]. These findings could further raise the hypothesis that neutrophils have a role to play in Ipilimumab and nivolumab related GI irAEs.
However, to validate the relationship between NLR and irAEs, continuous evaluation and monitoring from pre-treatment with ICIs to development of irAEs is recommended. A study by Matsukane et al. [32] showed that continuous monitoring of NLR trends may predict irAE onset and severity and subsequent prognosis. The NLR was significantly elevated during 4 weeks prior to irAEs especially in those who developed interstitial pneumonitis. Notably, patients who showed a prompt reduction in elevated NLRs had favorable progression-free survival (hazard ratio 0.32, 95% CI 0.10-1.01, p = 0.0140) and overall survival (hazard ratio 0.23, 95% CI 0.06-0.86, p = 0.0057) compared to the patients who maintained elevated NLRs [32].
In clinical trials
A study by Hong JY et al. [33] that was looking at characteristics of genomic properties of Hepatocellular Carcinoma (HCC) patients in response to anti-PD-1 immunotherapy in a single arm phase 2 trial, part of univariate analysis showed that low NLR was among clinicopathological features identified as contributing factors to pembrolizumab response. Meanwhile patients with progressive disease showed an increased number of both CD14+ and CD16+ monocytes and activation of neutrophil-associated pathways [33]. Additionally, a study to identify risk factors for hyper progression in advanced HCC patients treated with ICIs, revealed that elevated NLR was associated with hyper progression and lower survival rates [7]. The correlation between peripheral blood parameters and immune-checkpoint inhibitor efficacy in solid tumors, have also been investigated in phase 1 clinical trials, demonstrating that elevated Lactate Dehydrogenase (LDH) and dNLR were associated with poorer survival outcomes in patients treated with immunotherapy in phase I clinical trials, regardless of tumor type. These parameters represent an easy tool that might be considered as stratification factors in immunotherapy-based clinical trials [34].
In curative tumor resection
Prognostic value of NLR could be applied in determining prognosis prior to curative tumor resection. In a systematic review and meta-analysis of 31 studies comprising 7553 patients, evaluating the prognostic impact of pretreatment NLR in patients undergoing curative rectal cancer resection found that high NLR was associated with lower rate of pathologic complete response [35]. Similarly, in a retrospective study of 327 patients who underwent tumor-free margin resections for Adenocarcinoma of Gastro-Esophageal junction (AEG), NLR was significantly related to histology (P=0.035), pathological stage (P<0.0001) and tumor recurrence (P=0.022) [36].
Prognostic factor in pre & post treatment
The prognostic value of NLR is found to be predictive both in pre-treatment and post-treatment with ICIs. In a study of 175 patients with advanced NSCLC treated with nivolumab, a multivariate analysis showed a pretreatment neutrophil-tolymphocyte ratio (NLR)≥5 was independently associated with inferior OS (median 5.5 vs. 8.4 months; HR 2.07, 95% CI 1.3-3.3; p=0.002) and inferior PFS (median 1.9 vs. 2.8 months; HR 1.43, 95% CI 1.02-2.0; p=0.04) [37]. Another retrospective study of 54 patients with advanced NSCLC treated with anti-PD1 antibodies, was assessing NLR at baseline and 6 weeks post treatment showed that patients with a high post-treatment NLR (≥5) had significantly shorter Progression-Free Survival (PFS) than those with a low posttreatment NLR (median, 1.3 vs. 6.1 months, p < 0.001). High posttreatment NLR was independent prognostic factor for PFS and OS [38]. A study by Gibson et al. [39] exploring factors associated with early mortality (death from any cause within 60 days of initiating immunotherapy) identified high NLR to be among significant predictors of mortality [39-54]. More similar studies that looked at the association between NLR and patients’ outcomes are listed in Table 1.
Table 1:Summary of existing prognostic models incorporating circulating inflammatory cells..
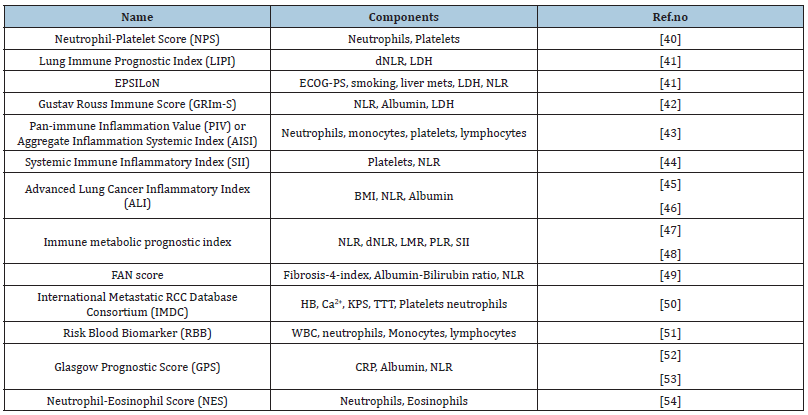
Abbreviations: BMI: Body Mass Index; Ca2+: Calcium Ions; CRP: C Reactive Protein; dNLR: Derived Neutrophil- Lymphocyte Ratio; ECOG-PS: Eastern Cooperative Oncology Group-Performance Status; HB: Hemoglobin; KPS: Karnofsky Performance Status; LDH: Lactate Dehydrogenase; LMR: Lymphocyte-Monocyte Ratio; NLR: Neutrophil-Lymphocyte Ratio; PLR: Platelet-Lymphocyte Ratio; TTT: Time to Treatment; WBC: White Blood Cells.
Incorporation in prognostic models
Various studies are ongoing to incorporate neutrophils in prognostic models. Matos et al. [55] developed a web tool called PIPO (phase 1 prognostic online), a user-friendly prognostic calculator for predicting Overall Survival (OS) and outcomes in patients to be included in phase 1 clinical trials with immune checkpoint Inhibitors (ICIs) or Targeted Agents (TAs) based on clinical parameters assessed at baseline. dNLR was among factors that showed significance calibration of OS. The overall accuracy of the model for 3-month OS prediction was 87.2% (95% CI 85%-90%). These results showed that PIPO could be a user-friendly objective and interactive tool to calculate specific survival probabilities for each patient before enrolment in a phase 1 clinical trial [55]. Other existing models are highlighted in Table 1. Likewise, another study that was looking at the Gustave Roussy Immune (GRIm)-Score Variation which takes into account NLR, serum albumin levels and Lactate Dehydrogenase (LDH) showed that variation in the score could be a more reliable peripheral blood biomarker of outcome in advanced Non-Small Cell Lung Cancer (NSCLC) patients treated with first-line pembrolizumab [56]. A similar study in advanced HCC patients treated with ICIs, confirmed that modified HCC-GRImscore system provided superior predictive ability in identifying patients who could benefit from ICIs as compared to original GRIm score and BCLC staging system [57].
A study by Sanchez Gastaldo et al. [51] created a risk score termed Risk Blood Biomarker score (RBB) which combines neutrophil-monocyte-lymphocyte ratio and white blood cells to stratify patients according to clinical outcome i.e treatment response and survival. Low RBB score with low baseline Lymphocyte-Monocyte Ratio (LMR), NLR and Platelet-Lymphocyte Ratio (PLR) were associated with better PFS and OS with improved survival outcomes and response to therapy [51]. Another review that was looking at circulating biomarkers in ICI treatment in lung cancer from liquid biopsies, mentioned the role of NLR, dNLR and Lung Immune Prognostic Index (LIPI). Although they are shown to be prognostic and predictive of outcome and survival but there are still unresolved questions regarding the difference in the magnitude of the benefit for patients treated with immunotherapy and the role of the LIPI score should be better defined in prospective studies [58]. Of note, dNLR can be longitudinally followed with a prognostic value of dNLR evolution during treatment in patients treated with ICI [10]
Relationship with other tumor markers
The prognostic role of neutrophils in combination with other tumor markers like ctDNA was analyzed in a European cohort of patients with resectable gastric cancer. The study found that CEA and CA 19-9 were independent prognostic factors for survival in a large cohort of patients with resectable gastric cancer. However, no relationship was found between tumor markers and ctDNA [59].
Platelets
Platelets are the smallest cells in the circulating blood with biconvex shape and no nucleus. They are derived from progenitor megakaryotic cell in bone marrow. They play a role in thrombosis and hemostasis of the circulatory system. It is established that platelets play a significant role in cancer growth and metastasis through a bidirectional interaction. Tumor and platelet aggregation protect cancer cells from immune surveillance through the crosslinking of integrins. Furthermore, platelets facilitate adhesion of tumor to vessel wall which enables extravasation of tumor cells and metastasis. Release of different growth factors, cytokines and chemokines enhance growth and angiogenesis [60].
Platelets as prognostiator for survival, treatment response and development of irAEs
The number of platelets was noted to have a clinical significance in cancer patients. It was seen that cancer patients had high platelet counts and the frequencies differed according to the type of cancer. Persistent high platelet count is associated with poor prognosis and shorter survival rate due to high risk of tumor invasion and distant metastasis [61,62]. A study in a Small Cell Lung Cancer (SCLC) model demonstrated the importance of platelet activation in promoting metastasis [63]. Like neutrophils, platelets are a surrogate inflammatory marker as they influence both innate and adaptive immune response. Interaction between platelets and lymphocytes is extensively studied. As low lymphocytes signify a state of impaired cellular immunity, the ratio between Platelets and Lymphocytes (PLR) has been evaluated as a prognostic marker in a number of solid tumors [2]. An elevated PLR indicates the activation of transcription factors of an inflammatory response, for example, the Signal Transducer and Activator of Transcription 3 (STAT3), Hypoxia-Inducible Factor 1a (HIF1a), and Nuclear FactorkB (NF-kB). These transcription factors result in the secretion of pro-inflammatory cytokines that also promote tumor growth, such as TNF-a, IL-1β, and IL-6. In addition, cancer-related inflammation plays a role in Epithelial–Mesenchymal Transition (EMT), angiogenesis, cell proliferation and survival, tumor–cell migration, invasion, and metastasis, as well as treatment response [61,64].
Incorporation in prognostic indices
Although high platelet count is regarded as prognostic marker for majority of tumors, studies have shown that their positive predictive value is high only when PLR is combined with other factors such as Hemoglobin/Albumin/Lymphocyte/Platelet (HALP) levels or Neutrophil/Platelet/Lymphocyte/Differentiation Score (NPLDS) which was assessed in predicting the prognosis of chemotherapeutic response in advanced gastric cancer. Collectively, the detection of PLR, HALP, and NPLDS are valuable although more prospective studies are needed for validation into clinical practice [61]. Table 1 provides other models that have included platelets as one of the parameters. Another study looked at the prognostic value of PLR in patients with unresectable metastatic colon cancer combined age, Alkaline Phosphatase (ALP), ascites and PLR to form the so-called AAAP scoring system. In this study, patients were classified into high, medium and low risk groups according to the score obtained. There were significant differences in OS between each group (P < 0.001). The study concluded that AAAP scoring system could be a useful predictive tool to aid in surgical decision making [65].
Likewise, a multicenter retrospective study that analyzed Neutrophil-platelet score (NPS), as a predictive systemic inflammation score for PD-1 Immune Checkpoint Inhibitors (ICI) in pretreated advanced Non-Small Cell Lung Cancer (NSCLC) patients showed that patients with high NPS had poor OS and more patients with progressive disease [66]. Data on liquid biomarkers with prognostic and predictive value in NSCLC treated with immunotherapy, shows that interaction of platelets with tumor cells in TME causes alteration of tumor cell RNA profiles which forms Tumor-Educated Platelets (TEPs). Furthermore, small nuclear RNAs were downregulated in TEPs hence this rich repertoire of RNA varieties could provide biomolecules for diagnostic and prognostic biomarkers [67]. In experimental mouse models and cancer cell lines, platelet count was found to correlate with resistance to chemotherapy. Activated platelets release growth factors and chemokines that counteract anti-proliferative and cytotoxic effects of chemotherapeutic agents and targeted therapies, upregulate the regulators of cell progression and enhance the phosphorylation of some of DNA repair proteins like Chk1, BRCA1 and Mre11 [68].
Utilizing platelet parameters
Other platelet parameters like Mean Platelet Volume (MPV) which measures the average size of platelets in the peripheral circulation also signifies the state of platelet activation. MPV or its related factors such as Platelet Distribution Width (PDW) are important in cancer progression. A study in metastatic Colorectal Cancer (CRC) [69] and NSCLC [70] revealed that decreased MPV and PLR were significantly associated with inferior OS. Therefore, studying their indices could be another effective mechanism in the diagnosis of several disorders including cancer [71].
Interaction of platelets with PDL1 as cancer survival mechanism
Multiple studies have demonstrated the interaction of tumor cells and platelets as a cancer survival mechanism [72]. Interaction between platelets and cancer cells enables transfer of PDL1 protein from cancer cells to the platelets. This transfer is mediated by fibronectin and other glycoproteins [73]. Asgari et al. [73], demonstrated that platelet derived factors Vascular-Endothelial Growth Factor (VEGF) and Platelet-Derived Growth Factor (PDGF) promote cancer cell PDL1 expression which in turn enables cancer cells to suppress immune cell activation [73]. Additionally, Notch, VEGF, PI3K/AKT, MAPK, NF-kB and STAT3 which are linked to platelet activation are known to activate PD-1 or PD-L1 for T cell regulatory cell expansion [74].
Platelets as a potential marker of response to ICIs
In the continuous efforts to identify other surrogate markers that could predict efficacy to ICIs, tumor-educated platelets derived PD-L1 mRNA was shown to be a surrogate biomarker predicting the PFS and OS of immunotherapy in patients with advanced NSCLC [75]. A study by Riesenberg et al. [76] demonstrated that there is an association between platelet count and efficacy of ICI. In this study, a preclinical model was used to study the role of platelets in T cell exhaustion while patient samples were used to evaluate the effect on response to ICI. The study revealed that there is a strong association between platelets and failure of ICI in both the preclinical and clinical settings, likely via modifying the amount of active tumor infiltrating CD8 T cells [76].
An algorithm developed to calculate activation of independent adjusted PDL1 payload of platelets (pPDL1Adj) by using NSCLC cell lines, was found to be superior to standard histological quantification from biopsies. These findings could help to overcome limitations of histological quantification of heterogeneous intratumoral PDL1 expression [72]. Several other studies have evaluated the interaction between platelets and leukocytes and how it could be used to predict treatment efficacy of ICIs. Anguera et al. [77] found that the proportion of monocytes with bound platelets (CD14+CD41+/ total monocytes) was significantly higher in patients with response to nivolumab than those with stable or progressive disease in NSCLC (p=0.002). Hence it was concluded that the functional modification induced by the platelet binding to the monocytes seems to be beneficial for the clinical response to ICI [77].
The role of platelets in immunity, inflammation and irAEs
Platelet-leucocyte complex was noted to contribute not only to inflammation and autoimmune disease but also have an immunoregulatory effect [78]. Molecules involved in plateletleukocyte complex include interaction between p-selectin-PSGL-1 and CD40-CD40L. Platelet binding and release of cytokines and growth factors like TGF-βdecrease T cell proliferation and production of inflammatory cytokines hence promoting immunoregulatory effect. Notably, platelet-monocyte complex was found to be higher in patients with systemic inflammation and Acquired Immunodeficiency Disease (AID) [79]. A similar study that used single RNA sequencing to identify immune cell types and biomarkers associated with irAEs, found that irAEs were associated with acute increase in monocytes and decrease in T cells [80]. In patients with Ulcerative Colitis (UC), it was found that onset flare was associated with lowest levels of CD14+PLT+. Membrane CD162 which is crucial for platelet binding was also downregulated only in monocytes (CD14+) from UC patients demonstrating the role of platelet-monocyte binding favoring inflammatory reaction [81].
In patients with Rheumatoid Arthritis (RA), the effect of platelet-lymphocyte binding was found to effectively regulate T lymphocyte function hence this mechanism could be applied to reduce inflammatory response in RA patients and provide a drug development concept for such debilitating diseases [82]. Interestingly, even though platelet-leucocyte interaction was shown to contribute to inflammation, AID and increasing risk of irAEs, this was not reflected in thromboembolic events caused by ICIs. In a study to identify risk factors associated with Cancer- Associated Thrombosis (CAT) in patients treated with ICIs, it was shown that a history of Venous Thromboembolism (VTE) or Arterial Thromboembolism (ATE), heart disease, antiplatelet and anticoagulant therapy were risk factors for CAT incidence. The identified risk factors were similar to the ones in Khorana score. However, Khorana score was designed for patients under chemotherapy. The currently modified Vienna score which includes levels of D-dimer and P-selectin is more predictive [83]. Thrombotic events reported in patients treated with ICIs happen as the consequence of T cell activation which induce the release of Thrombotic Factor (TF) derived from PDL1-monocytes. In addition, it was noted that IL-8 and other inflammatory cytokines were elevated in patients with ICI who developed thromboembolism. IL-8 induce platelet activation and spread through attracting more MDSCs into the tumor. MDSCs can promote platelet aggregation. Also, through CXCR1/MDSCs, induce tumor to release Neutrophil Extracellular Traps (NETs) which play a significant role in thrombosis [84].
Eosinophils
Eosinophils constitute a subpopulation of cells that originate from myeloid stem cells. Through activation of IL-5, they differentiate into eosinophil progenitor which finally gives rise to mature eosinophils. Phenotypically they can be identified by their bilobed nuclei and pink cytoplasmic granules. Circulating eosinophils are recruited into loose connective tissues especially in respiratory tract and gastrointestinal tract [1]. Eosinophils are involved in elimination of extracellular parasites like helminthes and also form a significant part of type 1 hypersensitivity reactions like asthma and atopic dermatitis. They function through degranulation where they release different cytokines that can be cytotoxic to viruses and bacteria. Also released cytokines recruit other white blood cell populations [3].
Apart from physiological and homeostatic functions, eosinophils are involved in pathogenic conditions like cancer where they display both pro-tumor and anti-tumor activity depending on the stimuli in the TME [3]. Also they are involved in immune stimulation due to their significant role in immune-mediated disorders, hypersensitivity reactions, leukocyte activation such as CD8+ and CD4+ T cells. Dominant immunostimulating function favours antitumor activity as demonstrated in the analysis of colorectal TME models which showed infiltrative eosinophils in growing tumors. Eosinophils were required for T cell activation in TME and therefore depleting eosinophils in TME, resulted to impaired T Helper Type 1 (Th1) responses and enhanced tumor burden in the model of intestinal tumorigenesis [85-88]. GM-CSF signal drive Interferon Regulatory Factor 5 (IRF5) activation in eosinophils. IRF5 regulate pro-inflammatory gene expression in myeloid cells and is a critical regulator of eosinophil activity in TME [88].
Eosinophils as prognostic surrogate for survival, treatment response and development of irAEs
Eosinophils are among inflammatory cells that have shown to be useful prognostic marker in several tumors, predicitive marker of treatment response particularly in immunotherapy and risk factor of developing irAEs [18,20,89]. However it is not clear whether this observation can be generalized as available literature has based this correlation mainly in melanoma and NSCLC. The prognostic role of eosinophils was related to Tumor-Associated Tissue Eosinophilia (TATE) or peripheral eosinophil counts [3]. In some tumors higher values were associated with better survival while others were not. This could be attributed to the cancer type, pathophysiology and origin of cancer, different measures of eosinophil count just to mention a few [3]. Generally, in determining the predicitve role it was found out that an early increase of eosinophils from baseline levels was associated with better response. In comparing responders to non-responders, the latter were found to have lower eotaxin-1 a stimulant of eosinophils, hence lower counts [3].
A study that compared lymphocytes, eosinophils and neutrophils counts in melanoma patients treated with ICIs, showed that those who responded to treatment had increased lymphocyte and eosinophil count at week 3 and week 6, and lower neutrophil count [90]. In a study of 259 patients with NSCLC treated with ICI therapy, peripheral eosinophil counts and percentages at the time of each ICI administration were evaluated from the beginning of ICI treatment up to Time to Treatment Failure (TTF). Univariable and multivariable analyses were performed to identify clinical factors associated with TTF. Eosinophil percentage of 5% or more and an eosinophil count of 330/μl or more within 6 weeks of ICPI therapy initiation was among significant favorable factors for prolonged TTF. Besides, percentage of eosinophils was a more useful way of analyzing than Absolute Eosinophil Count (AEC) [91]. Another retrospective study was looking at the prognostic role of eosinophilia and timing of eosinophilia on ICI treatment in advanced melanoma patients found that eosinophilia-on-ICI was an independent prognosticating factor for median OS (HR :0.223; 95% CI: 0.088-0.567; p = 0.002). ‘Late eosinophilia’ (≥1 year from ICI start date) group had better median OS (31.9 vs 24.1 vs 13.0 months; p = 0.002) when compared with ‘early eosinophilia’ (<1 year from IO start date) and ‘no eosinophilia’ groups, respectively. From these results it was proposed that inducing eosinophilia at a cetain time interval could enhance prolonged therapeutic benefit of ICIs [92]. The hypothesis behind positive correlation of role of timing of peak eosinophilia could be related to the fact that eosinophils prevent acquired resistance to ICI due to loss of tumor antigen expression and T cell exhaustion. In this scenario, they act as Antigen Presenting Cells (APCs), secrete chemo-attracts which help to recruit new T lymphocytes hence boosting the immune system [92].
Another role of eosinophils in promoting ICI treatment was demonstrated in a study by Zheng et al. [93]. Using a breast cancer model they showed that CTLA4 blockade promotes vessel normalization in breast tumors via the accumulation of eosinophils. Eosinophil accumulation was positively correlated with the responsiveness of a breast tumor to anti-CTLA4 therapy. Depletion of eosinophils subsequently negated vessel normalization, reduced antitumor immunity and attenuated tumor growth inhibition by anti-CTLA4 therapy. Therefore, these results demonstrated the role of eosinophils in vessel remodeling which augments efficacy of anti-CTLA4 therapy [93]. Improving hypoxia by normalizing blood vessels and improving radiosensitivity by immunotherapy has emerged as a new application of combined immunotherapy and radiotherapy. Interferon γ produced by CD4 + /CD8 + T cells, induced by immune checkpoint inhibitors, plays an important role in the normalization of blood vessels; tumor-associated eosinophils also play a role in the process of immunotherapy-induced blood vessel normalization. In addition, the reduction in regulatory T cells induced by immune checkpoint inhibitors can increase eosinophil levels, which promotes the further development of vascular normalization mechanisms [94].
Eosinophils and association with the development of irAEs
Despite the improving efficacy of ICIs, eosinophils have been linked to a number of cutaneous and organ specific irAEs [95]. A logistic regression analysis by Nakamura et al. [96], revealed that baseline absolute eosinophil count was positively associated with occurrence of endocrine irAEs (OR: 1.601, P = 0.045, cutoff value = 240/μL). Additionally, a higher relative eosinophil count at 1 month was significantly correlated with occurrence of endocrine irAEs (OR: 1.229, P = 0.0296, cutoff value = 3.2%) [96]. A similar retrospective study by Takayasu et al looked at the clinical features of ICI-induced secondary Adrenal Insufficiency (AI) that can be used for screening in standard clinical practice. Regression analysis showed a significant correlation between ICI-induced secondary AI and absolute or relative eosinophil counts at pre-onset of AI, as well as differences or rate of increase in eosinophil counts at baseline and at pre-onset. Absolute eosinophil counts >198.36/μL or relative eosinophil counts >5.6% at pre-onset, and a difference of 65.25/μL or a rate of eosinophil count increase of 1.97 between baseline and at pre-onset showed best sensitivity and specificity [97]. However the relationship between eosinophils and endocrine irAEs is thought to be due to destruction of adrenal gland and pituitary glands which leads to reduced glucocorticoid levels hence an increase in blood eosinophils [96].
Likewise a study of 300 patients with NSCLC treated with ICI was looking at the role of eosinophils in ICI Induced Pneumonitis (IIP) found out that 54 patients (18%) experienced ICI-pneumonitis and had a high level of baseline peripheral-blood Absolute Eosinophil Count (AEC) than those without ICI-pneumonitis (P=0.013). The incidence of ICI-pneumonitis was higher in the high-AEC group than in the low-AEC group (P<0.001). Moreover, patients with high AEC had a higher Objective Response Rate (ORR) (40.9% versus 28.8%, P=0.029) and longer PFS (8.93 months versus 5.87 months, P=0.038) [85]. In a review by Zen et al. [98] in analyzing mechanisms behind ICI-induced liver injury which is considered a new form of liver disease emerging in the era of cancer immunotherapy noted that mainly lymphocytes and occasional eosinophils infiltrated the liver resulting to liver injury [99-106] (Table 2).
Table 2:Summary of inflammatory cells and their role in immunotherapy..

Abbreviations: ALC: Absolute Lymphocyte Count; aGC: Advanced Gastric Cancer; aHCC: Advanced Hepatocellular Carcinoma; aNSCLC: Advanced Non-Small Cell Lung Cancer; AAAP: Age/Alkaline Phosphatase/Ascites/PLR; AID: Autoimmune Disease; CRC: Colorectal Cancer; CRP: C Reactive Protein; dNLR: Derived Neutrophil Lymphocyte Ratio; E-on- IO: Eosinophil on ICI; G3/G4 irAEs: Grade 3,4 Immune-Related Adverse Events; GI: Gastroinstestinal; GRIm score: Gustav Rouss Immune Score; HALP levels: Hemoglobin/Albumin/Lymphocyte/Platelet; HCC: Hepatocellular Carcinoma; ICIs: Immune Checkpoint Inhibitors; Io: Immun-Oncology; LDH: Lactate Dehydrogenase; MACE: Mace- Major Adverse Cardiac Events; MPV: Mean Plateletvolume; mets-metastasis; NLR: Neutrophil to Lymphocyte Ratio; NPLDS: neutrophil/platelet/lymphocyte/differentiation score; NPS: Neutrophil-Platelet Score; OS: Overall Survival; pCR-pathological complete response; PS: Performance Status; P1PO: Phase 1 Prognostic Online; PCT: Plateletcrit; PDW: Platelet Distribution Width; PLR: Platelet-Lymphocyte Ratio; PLTs: Platelets; PD: Progressive Disease; PFS: Progression Free Survival; PDL1: Programmed Death Ligand 1; RR: Recurrence Rate; RLC: Relative Lymphocyte Count; REC: Relative Eosinophil Count; RLC: Relative Lymphocyte Count; RBB: Risk Blood Biomarker; TTF: Time to Treatment Failure; TILs: Tumor Infiltrating Lymphocytes; WBCs: White Blood Cells.
Conclusions, Perspectives
It is evident that inflammatory immune cells have the potential of predicting efficacy of immune checkpoint inhibitors and risk of developing irAE. However most studies were done retrospectively, from single centres and with small sample sizes. As such it is not easy to conclude whether the observations can be generalized. Development of irAEs is a time bound event, affected by survival and duration of treatment. Those with longer survival and treated for a longer time are more likely to experience treatment related toxicities than those with shorter survival or short treatment exposure. Hence cumulative incidence analysis would be more appropriate to evaluate risk factors of developing irAEs than conventional multivariate analysis which was used in most of the observational studies. In addition, determination of cut-off values that are prognostic and indicative and mode of application of these ratios is something that would have to be addressed in future prospective studies to validate these values before application in daily clinical practice.
Acknowledgement
The authors wish to acknowledge Dr. Anne Nyambura Njogu for her technical support in the preparation of this manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pecorino L (2016) Molecular biology of cancer: Mechanisms, targets, and therapeutics. Oxford Univ Press, USA.
- Ravindranathan D, Master VA, Bilen MA (2021) Inflammatory markers in cancer immunotherapy. Biology 10(4): 325.
- Simon SCS, Utikal J, Umansky V (2019) Opposing roles of eosinophils in cancer. Cancer Immunol, Immunother 68(5): 823-833.
- Abdihamid O, Omar A, Rugambwa T (2021) Defining the correlation between immune-checkpoint inhibitors-related adverse events and clinical outcomes: A narrative review. Ecancermedicalscience 15: 1314.
- Liu X, Shi Y, Zhang D, Zhou Q, Liu J, et al. (2021) Risk factors for immune-related adverse events: What have we learned and what lies ahead? Biomark Res 9(1): 79.
- Wang Y, Tong Z, Zhang W, Zhang W, Buzdin A, et al. (2021) FDA-approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front Oncol 11: 683419.
- Kim CG, Kim C, Yoon SE, Kim KH, Choi SJ (2021) Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol 74(2): 350-359.
- Frelaut M, du Rusquec P, de Moura A, Le Tourneau C, Borcoman E (2020) Pseudoprogression and Hyperprogression as new forms of response to immunotherapy. BioDrugs 34(4): 463-476.
- Buchbinder EI, Desai A (2016) CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am J Clin Oncol 39(1): 98-106.
- Duchemann B, Remon J, Naigeon M, Mezquita L, Ferrara R, et al. (2020) Integrating circulating biomarkers in the immune checkpoint inhibitor treatment in lung cancer. Cancers 12(12): 3625.
- Zhang Y, Zhang X, Li W, Du Y, Hu W, et al. (2022) Biomarkers and risk factors for the early prediction of immune-related adverse events: a review. Hum Vaccin Immunother 18(1): 1-12.
- Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, et al. (2017) Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci 88(2): 225-231.
- Yang F, Wang JF, Wang Y, Liu B, Molina JR (2021) Comparative analysis of predictive biomarkers for PD-1/PD-L1 inhibitors in cancers: Developments and challenges. Cancers 14(1): 109.
- Michailidou D, Khaki AR, Morelli MP, Diamantopoulos L, Singh N (2021) Association of blood biomarkers and autoimmunity with immune related adverse events in patients with cancer treated with immune checkpoint inhibitors. Sci Rep 11(1): 9029.
- Ménétrier-Caux C, Ray-Coquard I, Blay JY, Caux C (2019) Lymphopenia in cancer patients and its effects on response to immunotherapy: An opportunity for combination with cytokines? Journal for immunotherapy of cancer 7(1): 85.
- Spassova I, Ugurel S, Kubat L, Zimmer L, Terheyden P, Mohr A, et al. (2022) Clinical and molecular characteristics associated with response to therapeutic PD-1/PD-L1 inhibition in advanced Merkel cell carcinoma. J Immunother Cancer 10(1): e003198.
- Paijens ST, Vledder A, de Bruyn M, Nijman HW (2021) Tumor-infiltrating lymphocytes in the immunotherapy era. Cellular & molecular immunology 18(4): 842-859.
- Isono T, Kagiyama N, Takano K, Hosoda C, Nishida T, et al. (2021) Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer 12(2): 153-164.
- Bai R, Li L, Chen X, Chen N, Song W, et al. (2021) Correlation of peripheral blood parameters and immune-related adverse events with the efficacy of immune checkpoint inhibitors. Journal of oncology 2021: 9935076.
- Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA (2017) Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget 8(69): 114268-114280.
- Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, et al. (2019) Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol 5(3): 376-383.
- Walsh MJ, Dougan M (2021) Checkpoint blockade toxicities: Insights into autoimmunity and treatment. Semin Immunol 52: 101473.
- Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ (2017) Neutrophils in cancer: Prognostic role and therapeutic strategies. Mol Cancer 16(1): 137.
- Valadez-Cosmes P, Maitz K, Kindler O, Raftopoulou S, Kienzl M (2021) Identification of novel low-density neutrophil markers through unbiased high-dimensional flow cytometry screening in non-small cell lung cancer patients. Front Immunol 12: 703846.
- Fujimoto A, Toyokawa G, Koutake Y, Kimura S, Kawamata Y, et al. (2021) Association between pretreatment neutrophil-to-lymphocyte ratio and immune-related adverse events due to immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac Cancer 12(15): 2198-2204.
- Lee PY, Oen KQX, Lim GRS, Hartono JL, Muthiah M, et al. (2021) Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers 13(6): 1308.
- Saini M, Szczerba BM, Aceto N (2019) Circulating tumor cell-neutrophil tango along the metastatic process. Cancer research 79(24): 6067-6073.
- Yang T, Hao L, Yang X, Luo C, Wang G, et al. (2021) Prognostic value of derived neutrophil-to-lymphocyte ratio (dNLR) in patients with non-small cell lung cancer receiving immune checkpoint inhibitors: A meta-analysis. BMJ open 11(9): e049123.
- Viñal D, Gutierrez-Sainz L, Martinez D, Garcia-Cuesta JA, Pedregosa J, et al. (2021) Prognostic value of neutrophil-to-lymphocyte ratio in advanced cancer patients receiving immunotherapy. Clin Transl Oncol 23(6): 1185-1192.
- Drobni ZD, Zafar A, Zubiri L, Zlotoff DA, Alvi RM, et al. (2020) Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor-associated myocarditis. J Am Heart Assoc 9(23): e018306.
- Shahabi V, Berman D, Chasalow SD, Wang L, Tsuchihashi Z, et al. (2013) Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med 11: 75.
- Matsukane R, Watanabe H, Minami H, Hata K, Suetsugu K, et al. (2021) Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Scientific reports 11(1): 1324.
- Hong JY, Cho HJ, Sa JK, Liu X, Ha SY, et al. (2022) Hepatocellular carcinoma patients with high circulating cytotoxic T cells and intra-tumoral immune signature benefit from pembrolizumab: Results from a single-arm phase 2 trial. Genome medicine 14(1): 1.
- Criscitiello C, Marra A, Morganti S, Zagami P, Viale G (2020) Pretreatment blood parameters predict efficacy from immunotherapy agents in early phase clinical trials. Oncologist 25(11): e1732-e1742.
- Hamid HKS, Davis GN, Trejo-Avila M, Igwe PO, Garcia-Marín A (2021) Prognostic and predictive value of neutrophil-to-lymphocyte ratio after curative rectal cancer resection: A systematic review and meta-analysis. Surg Oncol 37: 101556.
- Yuan D, Zhu K, Li K, Yan R, Jia Y, et al. (2014) The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J Surg Oncol 110(3): 333-340.
- Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, et al. (2017) Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung cancer 106: 1-7.
- Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, et al. (2018) Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 67(3): 459-470.
- Gibson A, Meyers D, Dean M, Stukalin I, Morris D, et al. (2021) P16.03 Early mortality associated factors with immune checkpoint inhibition in real-world Canadian NSCLC patients. Journal of Thoracic Oncology 16(3): S348.
- Mielgo Rubio X, Sereno Moyano M, Chara LE, López-Castro R, Rubio-Martínez J, et al. (2018) Neutrophil-Platelet Score (NPS), a predictive systemic inflammation score for PD-1 Immune Checkpoint Inhibitors (ICI) in pretreated advanced Non-Small Cell Lung Cancer (NSCLC) patients. Annals of Oncology 29(8): VIII510.
- Zhao Q, Li B, Xu Y, Wang S, Zou B, et al. (2021) Three models that predict the efficacy of immunotherapy in Chinese patients with advanced non-small cell lung cancer. Cancer Med 10(18):6291-6303.
- Lucy XM, Nicholas TH, Yifan W, Stephanie R, Michael JA, et al. (2020) Prognostic ability of the Gustave Roussy Immune Score for patients with advanced pancreatic adenocarcinoma. Journal of Clinical Oncology 40(4).
- Ginesu GC, Paliogiannis P, Feo CF, Cossu ML, Scanu AM, et al. (2022) Inflammatory indexes as predictive biomarkers of postoperative complications in oncological thoracic surgery. Curr Oncol 29(5): 3425-3432.
- Liu J, Li S, Zhang S, Liu Y, Ma L, et al. (2019) Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal 33(8): e22964.
- Dusselier M, Deluche E, Delacourt N, Ballouhey J, Egenod T, et al. (2019) Neutrophil-to-lymphocyte ratio evolution is an independent predictor of early progression of second-line nivolumab-treated patients with advanced non-small-cell lung cancers. PLoS One 14(7): e0219060.
- Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, et al. (2018) Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med 7(1): 13-20.
- Bauckneht M, Genova C, Rossi G, Rijavec E, Dal Bello MG, et al. (2021) The role of the immune metabolic prognostic index in patients with non-small cell lung cancer (NSCLC) in radiological progression during treatment with nivolumab. Cancers (Basel) 13(13): 3117.
- Castello A, Toschi L, Rossi S, Mazziotti E, Lopci E (2020) The immune-metabolic-prognostic index and clinical outcomes in patients with non-small cell lung carcinoma under checkpoint inhibitors. J Cancer Res Clin Oncol 146(5): 1235-1243.
- Kawashima A, Yamamoto Y, Sato M, Nakata W, Kakuta Y, et al. (2021) FAN score comprising fibrosis-4 index, albumin-bilirubin score and neutrophil-lymphocyte ratio is a prognostic marker of urothelial carcinoma patients treated with pembrolizumab. Sci Rep 11(1): 21199.
- Zheng W, Zhu W, Yu S, Li K, Ding Y et al. (2020) Development and validation of a nomogram to predict overall survival for patients with metastatic renal cell carcinoma. BMC Cancer 420(1): 1066.
- Sánchez-Gastaldo A, Muñoz-Fuentes MA, Molina-Pinelo S, Alonso-García M, Boyero L, et al. (2021) Correlation of peripheral blood biomarkers with clinical outcomes in NSCLC patients with high PD-L1 expression treated with pembrolizumab. Transl Lung Cancer Res 10(6): 2509-2522.
- Namikawa T, Yokota K, Tanioka N, Fukudome I, Iwabu J et al. (2020) Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today 50(11):1486-1495.
- Yamamoto T, Ito T, Hase T, Ishigami M, Mizuno K et al. (2022) Immune-related liver injury is a poor prognostic factor in patients with nonsmall cell lung cancer treated with immune checkpoint inhibitors. Cancer Invest 40(2): 189-198.
- Tucker MD, Brown LC, Chen YW, Kao C, Hirshman N, et al. (2021) Association of baseline neutrophil-to-eosinophil ratio with response to nivolumab plus ipilimumab in patients with metastatic renal cell carcinoma. Biomark Res 39(1): 80.
- Matos I, Villacampa G, Hierro C, Martin-Liberal J, Berché R, et al. (2021) Phase I prognostic online (PIPO): A web tool to improve patient selection for oncology early phase clinical trials. Eur J Cancer 155: 168-178.
- Lenci E, Cantini L, Pecci F, Cognigni V, Agostinelli V, et al. (2021) The Gustave Roussy Immune (GRIm)-Score Variation Is an Early-on-Treatment Biomarker of Outcome in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients Treated with First-Line Pembrolizumab. J Clin Med 10(5): 1005.
- Li Y, Pan Y, Lin X, Hou J, Hu Z, et al. (2021) Development and validation of a prognostic score for hepatocellular carcinoma patients in immune checkpoint inhibitors therapies: The hepatocellular carcinoma modified gustave roussy immune score. Front Pharmacol 12: 819985.
- Sorich MJ, Rowland A, Karapetis CS, Hopkins AM (2019) Evaluation of the lung immune prognostic index for prediction of survival and response in patients treated with atezolizumab for NSCLC: Pooled analysis of clinical trials. J Thorac Oncol 14(8): 1440-1446.
- Slagter AE, Vollebergh MA, Caspers IA, van Sandick JW, Sikorska K, et al. (2022) Prognostic value of tumor markers and ctDNA in patients with resectable gastric cancer receiving perioperative treatment: Results from the CRITICS trial. Gastric Cancer 25(2): 401-410.
- McNicol A, Israels SJ (2008) Beyond hemostasis: The role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targets 8(2): 99-117.
- Yu L, Guo Y, Chang Z, Zhang D, Zhang S, et al. (2021) Bidirectional interaction between cancer cells and platelets provides potential strategies for cancer therapies. Front Oncol 11: 764119.
- Liu Y, Zhang Y, Ding Y, Zhuang R (2021) Platelet-mediated tumor metastasis mechanism and the role of cell adhesion molecules. Crit Rev Oncol Hematol 167: 103502.
- Hyslop SR, Kersbergen A, Alexander WS, Sutherland KD, Josefsson EC (2019) Exploring immunotherapy and the role of platelets in a murine model of metastatic small-cell lung cancer. Research and Practice in Thrombosis and Haemostasis 3: 566.
- Liu D, Czigany Z, Heij LR, Bouwense SAW, van Dam R, et al. (2022) The value of platelet-to-lymphocyte ratio as a prognostic marker in cholangiocarcinoma: A systematic review and meta-analysis. Cancers 14(2): 438.
- Li ZM, Peng YF, Du C, Gu J (2016) Colon cancer with unresectable synchronous metastases: the AAAP scoring system for predicting the outcome after primary tumour resection. Colorectal Dis 18(3): 255-263.
- Honrubia PB, Garde NJ, García SJ, Piera MN, Llombart CA, et al. (2021) Soluble biomarkers with prognostic and predictive value in advanced non-small cell lung cancer treated with immunotherapy. Cancers 13(17): 4280.
- Braun A, Anders HJ, Gudermann T, Mammadova BE (2021) Platelet-cancer interplay: Molecular mechanisms and new therapeutic avenues. Frontiers in oncology 11: 665534.
- Chang J, Lin G, Ye M, Tong D, Zhao J, et al. (2019) Decreased mean platelet volume predicts poor prognosis in metastatic colorectal cancer patients treated with first-line chemotherapy: Results from mCRC biomarker study. BMC cancer 19(1): 15.
- Omar M, Tanriverdi O, Cokmert S, Oktay E, Yersal O, et al. (2018) Role of increased mean platelet volume (MPV) and decreased MPV/platelet count ratio as poor prognostic factors in lung cancer. The clinical respiratory journal 12(3): 922-929.
- Halawi M (2022) Prognostic value of evaluating platelet role, count and indices in laboratory diagnosis of different types of solid malignancies. Pakistan journal of biological sciences 25(2): 100-105.
- Lesyk GM, Poitras E, Jurasz P (2018) Effect of platelets and their pharmacological regulation on cancer cell immune checkpoint PD-l1 expression. FASEB Journal 32(1).
- Hinterleitner C, Strähle J, Malenke E, Hinterleitner M, Henning M, et al. (2021) Platelet PD-L1 reflects collective intratumoral PD-L1 expression and predicts immunotherapy response in non-small cell lung cancer. Nature Communications 12(1): 7005.
- Asgari A, Lesyk G, Poitras E, Govindasamy N, Terry K, et al. (2021) Platelets stimulate programmed death-ligand 1 expression by cancer cells: Inhibition by anti-platelet drugs. Journal of thrombosis and haemostasis 19(11): 2862-2872.
- Lee DY, Im E, Yoon D, Lee YS, Kim GS, et al. Pivotal role of PD-1/PD-L1 immune checkpoints in immune escape and cancer progression: Their interplay with platelets and FOXP3+Tregs related molecules, clinical implications and combinational potential with phytochemicals. Seminars in cancer biology 86(3): 1033-1057.
- Chang J, Hu Z, Sun S, Yu H, Wu X, et al. (2019) P1.04-20 PD-L1 mRNA derived from tumor-educated platelets predicts the clinical outcome of immunotherapy in non-small cell lung cancer. Journal of Thoracic Oncology 14(10): S447.
- Riesenberg BP, Li M, Spakowicz D, Hoyd R, Beane J, et al. (2020) Platelets impact the responsiveness of immune checkpoint blockade therapy in solid tumors. Journal of Clinical Oncology 38 (15_suppl): e15023.
- Anguera G, Zamora C, Ortiz MA, Andres M, Vidal S, et al. (2017) P3.02c-080 The beneficial effect of platelet binding to monocytes on the clinical response to checkpoint inhibitors. Journal of Thoracic Oncology 12(1): S1326.
- Zamora C, Cantó E, Nieto JC, Ortiz MA, Diaz TC, et al. (2013) Functional consequences of platelet binding to T lymphocytes in inflammation. Journal of leukocyte biology 94(3): 521-529.
- Zamora C, Riudavets M, Anguera G, Alserawan L, Sullivan I, et al. (2021) Circulating leukocyte-platelet complexes as a predictive biomarker for the development of immune-related adverse events in advanced non-small cell lung cancer patients receiving anti-PD-(L)1 blocking agents. Cancer Immunology, Immunotherapy 70(6): 1691-1704.
- Chen J, Pflieger L, Grimes S, Baker T, Brems M, et al. (2020) Identify immune cell types and biomarkers associated with immune-related adverse events using single cell RNA sequencing. Journal for ImmunoTherapy of Cancer 8(Suppl 3): A39.
- Zamora C, Canto E, Nieto JC, Garcia PE, Gordillo J, et al. (2018) Inverse association between circulating monocyte-platelet complexes and inflammation in ulcerative colitis patients. Inflammatory bowel diseases 24(4): 818-828.
- Zamora C, Cantó E, Nieto JC, Bardina J, Diaz TC, et al. (2017) Binding of platelets to lymphocytes: A potential anti-inflammatory therapy in rheumatoid arthritis. Journal of immunology 198(8): 3099-3108.
- Ando Y, Hayashi T, Sugimoto R, Nishibe S, Ito K, et al. (2020) Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Investigational new drugs 38(4): 1200-1206.
- Zou XL, Chen WY, Zhang GY, Ke H, Yang QH, et al. (2021) Risk factors, incidence, and prognosis of thromboembolism in cancer patients treated with immune checkpoint inhibitors. Frontiers in pharmacology 12: 747075.
- Chu X, Zhao J, Zhou J, Zhou F, Jiang T, et al. (2020) Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung cancer 150: 76-82.
- Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L (2021) The crosstalk between tumor cells and the immune microenvironment in breast cancer: Implications for immunotherapy. Frontiers in oncology 11: 610303.
- Diny NL, Rose NR, Čiháková D (2017) Eosinophils in autoimmune diseases. Frontiers in immunology 8: 484.
- Arnold IC, Artola BM, Gurtner A, Bertram K, Bauer M, et al. (2020) The GM-CSF-IRF5 signaling axis in eosinophils promotes antitumor immunity through activation of type 1 T cell responses. The Journal of experimental medicine 217(12): e20190706.
- Okauchi S, Shiozawa T, Miyazaki K, Nishino K, Sasatani Y, et al. (2021) Association between peripheral eosinophils and clinical outcomes in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Polish Archives of Internal Medicine 131(2): 152-160.
- Ohashi H, Takeuchi S, Miyagaki T, Kadono T (2020) Increase of lymphocytes and eosinophils and decrease of neutrophils at an early stage of anti-PD-1 antibody treatment is a favorable sign for advanced malignant melanoma. Drug discoveries & therapeutics 14 (3): 117-121.
- Osawa H, Shiozawa T, Okauchi S, Miyazaki K, Kodama T, et al. (2021) Association between time to treatment failure and peripheral eosinophils in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Polish Archives of Internal Medicine 131(10): 16049.
- Kartolo A, Holstead R, Hopman W, Baetz T (2021) Prognosticating role of serum eosinophils on immunotherapy efficacy in patients with advanced melanoma. Immunotherapy 13(3): 217-225.
- Zheng X, Zhang N, Qian L, Wang X, Fan P, et al. (2020) CTLA4 blockade promotes vessel normalization in breast tumors via the accumulation of eosinophils. International journal of cancer 146(6): 1730-1740.
- Liu Z, Zhao Q, Zheng Z, Liu S, Meng L, et al. (2021) Vascular normalization in immunotherapy: A promising mechanisms combined with radiotherapy. Biomedicine pharmacotherapy 139: 111607.
- Ramos CM, Flores CA, Brito ZP (2021) Emerging role of eosinophils in immune-related adverse events related to therapy with immune checkpoint inhibitors. Pol Arch Intern Med 131(10): 16092.
- Nakamura Y, Tanaka R, Maruyama H, Ishitsuka Y, Okiyama N, et al. (2019) Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Japanese journal of clinical oncology 49 (5): 431-437.
- Takayasu S, Mizushiri S, Watanuki Y, Yamagata S, Usutani M, et al. (2022) Eosinophil counts can be a predictive marker of immune checkpoint inhibitor-induced secondary adrenal insufficiency: A retrospective cohort study. Scientific reports 12(1): 1294.
- Zen Y, Yeh MM (2019) Checkpoint inhibitor-induced liver injury: A novel form of liver disease emerging in the era of cancer immunotherapy. Seminars in diagnostic pathology 36(6): 434-440.
- Bai R, Chen N, Chen X, Li L, Song W, et al. (2021) Analysis of characteristics and predictive factors of immune checkpoint inhibitor-related adverse events. Cancer biology & medicine 18(4): 1118-1133.
- Alessi JV, Ricciuti B, Alden S, Awad M (2021) P14.21 baseline derived neutrophil-to-lymphocyte ratio (DNLR) and clinical outcomes to first-line pembrolizumab in NSCLC with high pd-l1 (≥50%). Journal of Thoracic Oncology 16(3): S338-S339.
- Laino AS, Woods D, Vassallo M, Qian X, Tang H, et al. (2020) Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. Journal for immunotherapy of cancer 8(1): e000842.
- Rose AAN, Kelly D, Hogg D, Butler MO, Saibil S, et al. (2020) 1144P Clinical predictors of therapeutic benefit from anti-PD1 immune checkpoint inhibitors (ICI) in patients (pts) with metastatic uveal melanoma. Annals of Oncology 31(4): S764-S765.
- Hopkins AM, Kichenadasse G, Garrett ME, Karapetis CS, Rowland A, et al. (2020) Development and validation of a prognostic model for patients with advanced lung cancer treated with the immune checkpoint inhibitor atezolizumab. Clinical Cancer Research 26(13): 3280-3286.
- Viscardi G, Sparano F, Di Liello R, Casal GA, Ferreres RB, et al. (2019) 86P-A novel Immunoscore, based on clinical and blood biomarkers, as prognostic model for immunotherapy in NSCLC. Annals of Oncology 30(11): xi31.
- Qi WX, Xiang Y, Zhao S, Chen J (2021) Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with first-line chemotherapy and atezolizumab. Cancer immunology immunotherapy 70(11): 3199-3206.
- Shimozaki K, SukawaY, Sato Y, Horie S, Chida A, et al. (2021) Analysis of risk factors for immune-related adverse events in various solid tumors using real-world data. Future oncology 17(20): 2593-2603.
© 2023 Tibera K Rugambwa. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






