- Submissions

Full Text
Gastroenterology Medicine & Research
Prognostic Value of The Lodds in The Probability of 5-Year Survival in Resecable Colorectal Carcinoma
Edgar Fermín Yan Quiroz1*, Carlos Estevan Chú Ramírez1, Renzo Nuñez Pacheco2, Folker Agreda Castro1 and José Richard Tenazoa Villalobos3
1Surgeon Oncologist, Assistant Physician of the High Complexity Hospital “Virgen de la Puerta”, Professor at the Faculty of Medicine of the Antenor Orrego Private University- Trujillo, Peru
2Surgeon Oncologist, Medical Assistant of the Hospital III EsSalud Juliaca Hospital, Peru
3Resident Physician of Oncological Surgery of the High Complexity Hospital “Virgen de la Puerta”, Trujillo, Peru
*Corresponding author: Edgar Fermín Yan Quiroz, Surgeon Oncologist, Assistant Physician of the High Complexity Hospital “Virgen de la Puerta”, Professor at the Faculty of Medicine of the Antenor Orrego Private University-Trujillo, Peru
Submission:December 22, 2022;Published: February 14, 2023

ISSN 2637-7632Volume7 Issue3
Abstract
Objective: To demonstrate the prognostic value of the LODDS in the probability of survival at 5 years in
resectable colorectal carcinoma
Material and Methods: Survival analysis of a consecutive series of 71 patients who underwent colectomy
for adenocarcinoma of the colon and upper rectum - middle, in the Oncological Surgery Service of the
High Complexity Hospital “Virgen de la Puerta” EsSalud de Trujillo - Peru during the years 2018-2019.
The follow-up was carried out until 06/01/2020.
Results: The mean age of the total series was 66.7 ± 15.1 years (range: 32 - 92 years). The male sex
(52.1%) predominated over the female (47.9%). The median time of illness was 7 months (range: 1.0
- 36 months). The 5-year actuarial survival rates in colorectal cancer patients were 94.1%, 93.2% and
80% for pN1, pN2 and pN3 respectively (p = 0.414). The 5-year actuarial survival rates in colorectal
cancer patients were 100%, 97.6%, and 80.7% for LODDS 0, LODDS 1, and LODDS 2, respectively (p =
0.414).
Conclusion: The LODDS has a prognostic value in actuarial survival at 5 years in patients with colorectal
carcinoma.
Keywords:LODDS; 5-year survival; Locorectal carcinoma
Introduction
Regional Lymph Node (LN) metastasis is the main predictor of outcome in postoperative treatment for patients with colorectal cancer [1]. In the eighth edition of the Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC) staging system for lymph Node Metastases (TNM), they are classified as pN1a (1 node metastatic), pN1b (2-3 metastatic nodes), pN2a (4-6 metastatic nodes) and pN2b (>7 metastatic nodes) according to the number of nodes involved [2]. Several observational studies [3-5] find that increased survival is associated with the evaluation of an adequate number of lymph nodes (≥12 resected nodes). However, a population-based analysis found that only 37% of colon cancer patients receive adequate lymph node evaluation [6]. Several factors can potentially influence lymph node dissection, including patient and tumor related factors, but also pathology and surgical technique; for example, adequate lymph node dissection is often reduced in men, the elderly, the obese, and patients of lower socioeconomic status, while larger and poorly differentiated tumors, more extensive operative specimens, and preoperative tumor markings are associated with an increased output or collection of lymph nodes [7]. It is suggested that a certain number of examined lymph nodes below 12 is associated with stage migration or understaging of the disease [8,9] thus resulting in inappropriate treatment. Therefore, new parameters, different and more precise than the classical pN staging, are necessary, since only a minimum number of resected lymph nodes is not enough, such as the number of negative lymph nodes [10], the number of lymph nodes involved [11], the proportion of lymph nodes involved and collected, also known as the lymph node ratio (LNR) [12] The node ratio (LNR) is the ratio of metastatic lymph nodes examined to the number of lymph nodes resected and appears to be less influenced by the total number of nodes dissected [13].
However, in node-negative patients, it does not provide a more meaningful prognostic assessment than TNM, because LNR0 is the same as the pN0 classification; therefore, N0 patients could not benefit from the classification system based on nodal ratio [14] As a result of these observations, the log probability of positive lymph nodes (LODDS) is proposed, which is defined as the logarithm of the ratio between the number of positive nodes and the number of negative nodes (adding the value plus 0.5, both for the numerator as for the denominator, to avoid null values mentioned above) [14- 16]. Another advantage of this LODDS system as opposed to the metastatic nodal ratio is that the LNR is based on the assumption that patients, within the same stratification, have the same prognosis, regardless of the number of lymph nodes examined. To illustrate this statement, consider two patients with clear prognostic differences (5/5 positive lymph nodes versus 30/30 positive lymph nodes). Both patients, based on the LNR, fall into the same stratification (LNR of 1), potentially introducing a prognostic misrepresentation of true events. LODDS obviates the inevitable product of singularities in the case that none or all of the collected lymph nodes are involved, and survival can still be estimated regardless of the number of lymph nodes examined. As a result, LODDS has been associated with a lower risk of stage migration in several neoplasms [5,6]. Thus, the LODDS classification can classify colon cancer patients into homogeneous groups regardless of the total number of lymph nodes examined [12]. Wang et al. [17] in a retrospective exploratory study based on the Surveillance Epidemiology End Results Program (SEER), work on a population registry sponsored by the National Cancer Institute, where they analyze a total of 24,477 stage IIIC colon cancer. Patients were subclassified into five groups, LODDS 1 to LODDS 5, according to the value of the ratio: LODDS1 (LODDS <−2.2); LODDS2 (−2.2 ≤ LODDS <−1.1); LODDS3 (−1.1 ≤LODDS <0); LODDS4 (0 ≤LODDS <1.1) and LODDS 5 (LODDS≥1.1). The 5-year survival rates for each of the groups respectively were 64.8%, 56%, 45.1%, 33.5% and 23.3%. These authors point out that the incorporation of LODDS into the colon cancer staging system allows clinicians to more accurately assess the prognosis of patients. They also advocate that the implementation of this system would constitute a common platform to compare inter-institutional and international treatment results. Scarinci et al. [14] evaluated 323 consecutive patients with a diagnosis of primary adenocarcinoma of the colon or rectum, who underwent curative resection in the Surgery Department of the Regina Apostolorum Hospital in Albano Laziale (Rome) between October 2010 and December 2010. 2015. Patients were divided into three groups: LODDS0 (≤ - 1.36), LODDS1 (> - 1.36 ≤ - 0.53) and LODDS2 (> - 0.53). These authors found 3-year overall survival rates of 88.3, 74.8, and 61.8% for each of the groups, respectively (p ≤ 0.001). Occhionorelli et al. [18] carry out a retrospective study at the Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Italy. They evaluated 210 patients who underwent urgent colonic resection for complicated cancer (occlusion, perforation, bleeding, sepsis) from 2003 to 2013. They grouped the patients into LODDS 0: −1.36 ≤; LODDS 1: > 1.36 - < 0.53 and LODDS 2 > 0.53. The calculated 5-year disease-free survival rates were 82.02, 73.75%, and 51.52% for each of the groups, respectively (p < 0.0001).
The deterioration of the prognosis of patients with lymph node metastases is related to the lack of regional and distant control of the disease. The prognostic capacity of regional lymph node involvement is usually complex as it depends on the primary location of the tumor, the lymph node dissection performed, and the number of lymph nodes resected, among others. Proper staging, which is what the following work intends, is to achieve an adequate N staging, thus allowing adequate prediction of patient survival, since it can be used as a finer stratification tool for the design of clinical trials and will allow selecting that patients should have a more specific and/or aggressive adjuvant regimen or strict surveillance as a result of our results applied to our regional reality.
Problem
What is the prognostic value of the LODDS in 5-year survival in patients diagnosed with colon adenocarcinoma operated on in the Oncological Surgery Service of the “Virgen de la Puerta” EsSalud High Complexity Hospital?
Research Objectives
General objective
To demonstrate the prognostic value of the LODDS in the probability of 5-year survival in resectable colorectal carcinoma
Specific objectives
A. Determine the 5-year survival rate of patients with LODDS 0
B. Estimate the 5-year actuarial survival rate of patients with
LODDS 1
C. To specify the 5-year survival rate of patients with LODDS 2
D. Compare the survival curves of all the groups described.
E. Estimate the actuarial survival at 5 years according to the
regional lymph node status (pN) of the AJCC classification.
Material and Methods
The present longitudinal and observational survival analysis study analyzed information from a consecutive series of 71 patients who underwent colectomy for adenocarcinoma of the colon and upper-middle rectum, in the Oncological Surgery Service of the High Complexity Hospital “Virgen de la Puerta” EsSalud de Trujillo - Peru during the years 2018-2019 (sample census).
Inclusion criteria
A. Patients of both sexes, older than and equal to 18 years with
anatomopathological diagnosis of adenocarcinoma of the
colon and rectum who underwent radical colectomy.
B. Patients with ECOG 0 – 2.
C. Patients with a tumor located between the cecum and the
upper rectum.
Exclusion criteria
A. Patients with distant metastases
B. Complications of the tumor (perforation and intestinal
obstruction at the time of the intervention)
C. Patients with previous chemotherapy or radiotherapy
D. Patients with synchronous or metachronous tumors
E. Patients pregnant or lactating women.
F. Patients who underwent surgery and initially in an institution
other than the Virgen de la Puerta EsSalud High Complexity
Hospital.
Operational definition of variables
Neoplasms were classified by stage using the criteria proposed by the American Committee Against Cancer (AJCC) (2). According to this classification, there must be histological confirmation of carcinoma and the following procedures are necessary to evaluate the categories: Primary tumor (T): physical examination, images, endoscopy, biopsy and/or surgical exploration; Regional lymph nodes (N): examination, imaging, and/or surgical exploration; and Distant metastasis (M): physical examination, imaging, and/or surgical exploration.
Log-positive lymph node probabilities (LODDS)
To calculate the LODDS, the following formula was considered:
Patients who met the selection criteria were included. The date
of surgery and the type of surgical intervention were verified. The
number of resected lymph nodes was evaluated in the pathology
result and grouped into positive and negative lymph nodes. The
formula is used to calculate the LODDS. According to his findings,
the patient will be stratified into (14) (18):
A. LODDS 0 (n = 43): ≤ − 1.36
B. LODDS 1 (n = 21): > - 1.36 - < - 0.53
C. LODDS 2 (n = 7): > - 0.53.
The 5-year actuarial survival rate was calculated according to the follow-up time from colorectal surgery to the established cutoff point or date of death. The patient’s status was also included if he is censored (alive) or deceased. Survival curves between LODDS 0, 1 and 2 were also compared.
5-year survival
It is the probability, expressed as a percentage, that a patient
remains alive after 5 years of being exposed to an event. A Kaplan
Meier statistical calculation method was used to obtain the observed
survival probability, using information given by individuals who
were followed for a time set by the investigator (see statistical
analysis plan).
A. Index: Censored / Deceased.
Information collection procedures
The data was recorded in a collection form that will include demographic data, clinical findings at the time of admission, auxiliary examinations (hematological, biochemical, including albumin values, ultrasound, endoscopic, biopsy, radiographic examinations), staging of the disease, type of surgery, date of operation, histopathological findings, location and size of the lesion, follow-up and patient status.
Data analysis plan
The Chi-square test or the two-tailed Fisher’s exact test were used to compare the categorical variables. The comparison of 2 arithmetic means was used the student’s “t” test. The 5-year actuarial survival percentages were estimated by the Kaplan Meier test. The present study will evaluate 71 patients who underwent colorectal surgery between July 1, 2018 and November 30, 2019, and for purposes of calculating the Kaplan Meier actuarial survival, a minimum follow-up period of 6 months was set (point of cutoff: June 1, 2020). For the comparison of 2 or more survival curves, the log rank test was used. The alternate hypothesis was accepted if the p value obtained is less than 0.05 (p < 0.05). The statistical packages SPSS v. 25.0.
Ethical aspects
In the present research work, the data of the patients under study was kept strictly confidential, it also only served for academic purposes. Before performing the surgical procedure, informed consent was obtained from each patient. It was approved by the Training, Research and Teaching Support Office of the Virgen de la Puerta High Complexity Hospital.
Results
Clinical characteristics of patients with resectable colorectal cancer
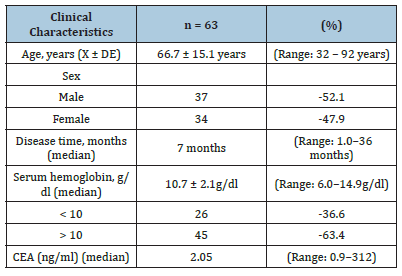
The average age of the total series was 66.7 ± 15.1 years (range: 32-92 years). The male sex (52.1%) predominated over the female (47.9%). The median length of illness was 7 months (range: 1.0-36 months). The mean serum hemoglobin was 10.7 ± 2.1 g/dl (range: 6.0-14.9 g/dl). 36.6% of the patients had hemoglobin <10g/dl. The median carcinoembryonic antigen (CEA) was 2.05 ng/dl (range: 0.9-312) (Table 1).
Table 1:Clinical characteristics of patients with resectable colorectal cancer.
Pathological characteristics of patients with resectable colorectal cancer/
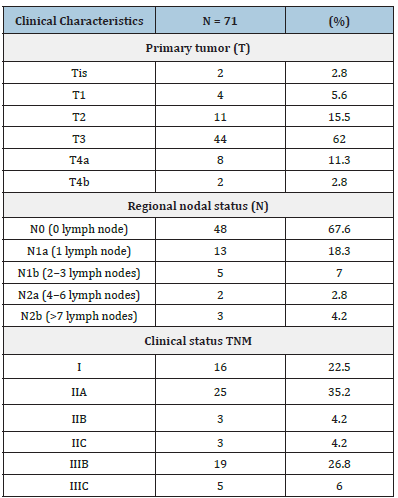
62% (n = 44) presented involvement of the subserosa (pT3), followed by involvement of the muscula propria (pT2) in 15.5% of the cases. Regarding the regional lymph node status (pN), 67.6% of the patients presented absence of lymph node metastasis, while 18.3% presented involvement of at least 1 lymph node (pN1a). The predominant pathological stage was type IIA (35.2%), followed by IIIB (26.8%) and stage I (22.5%) (Table 2).
Table 2:Pathological characteristics of patients with resectable colorectal cancer.
ROC curve (Receiver Operating Characteristic) for the LODDS
To assess the discriminative capacity of the LODDS in 5-year actuarial survival, we used the ROC curve. We found an area under the curve of 0.676 (95% CI: 0.474 - 0.877; p value = 0.192), that is, the LODDS is a test that regularly discriminates a patient with lymph node involvement with adequate survival from that with nodal involvement with inadequate survival (Figure 1).
Figure 1: ROC curve of LODDS in resectable colorectal carcinoma.
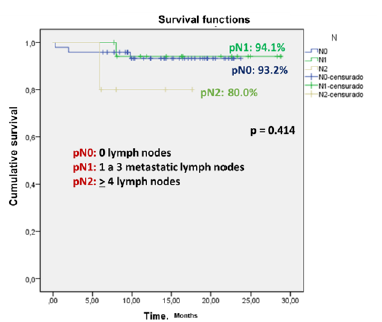
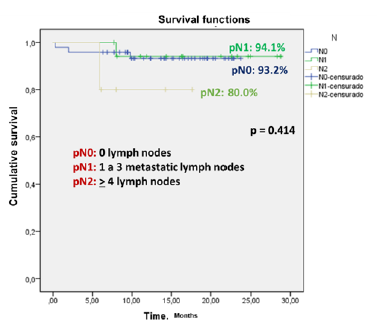
Actuarial 5-year survival in patients with colorectal cancer using the AJCC TNM system
The 5-year actuarial survival rates in patients with colorectal cancer were for pN1, pN2, and pN3 of 94.1%, 93.2%, and 80%, respectively (p = 0.414) (Figure 2).
Figure 2: Actuarial 5-year survival in patients with colorectal cancer using the AJCC TNM system.
Actuarial 5-year survival in patients with colorectal cancer using the LODDS model
The 5-year actuarial survival rates in patients with colorectal cancer were for LODDS 0, LODDS 1, and LODDS 2 of 100%, 97.6%, and 80.7%, respectively (p = 0.414) (Figure 2).
Discussion
Table 3:Main clinical and pathological characteristics of patients with resectable colorectal cancer according to LODDS.
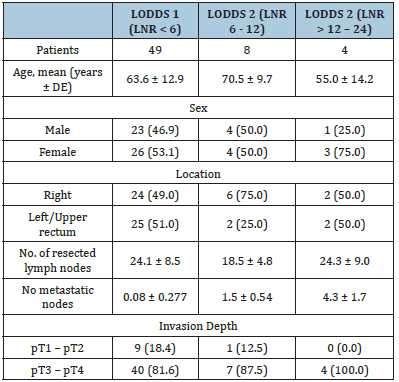
The LODDS showed greater prognostic significance compared to NELN. In addition, these data further support the literature on the clinical relevance of LODDS staging to overall survival, rather than that of LNR classification. This suggests that LODDS may detect some LN features missed when using another lymph node classification, suggesting that LODDS may be more useful. The results demonstrated that LODDS is an independent prognostic factor for survival, unlike TNM and LNR staging, which is similar to the literature in different surgical fields. In patients in which the number of LNs extracted was equal to the number of positive LNs, for example LNR1, or in cases with negative LNs, LNR is not able to stratify the sample and there is a risk of “migration stage”. This is because LODDS uses a mathematical approach to lymph node staging, which takes into account the number of negative LNs and is not influenced by the extent of lymphadenectomy; therefore, unlike the pN and LNR stage, LODDS represents the probability of an exam LN to be metastatic. We found that LODDS allows for better prognostic stratification of patients with colorectal cancer compared when using traditional TNM lymph node classification, suggesting that LODDS may be more useful (Table 3&4). The results demonstrated that LODDS is an independent prognostic factor for survival, unlike TNM and LNR staging, which is similar to the literature in different surgical fields. In patients where the number of LNs cleared was equal to the number of LN positives, for example LNR1, or in cases with LN negatives, LNR cannot stratify the sample and there is a risk of “migration stage”. This is because LODDS uses a mathematical approach to lymph node staging, which takes into account the number of negative LNs and is not influenced by the extent of lymphadenectomy; therefore, unlike the pN and LNR stage, LODDS represents the probability that an examined LN is metastatic. In our study, as shown in Figure 1, the 3-year overall survival decreases with increasing LODDS results: 85.1% in LODDS0, 72.8% in LODDS1, and 47.1% of LODDS2 groups. In multivariate analysis, the statistical significance of this variable provides the ability of LODDS to affect patient prognosis (Figure 3-5).
Table 4:
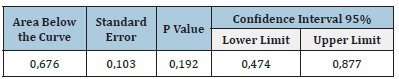
Figure 3: Actuarial 5-year survival in patients with colorectal cancer using the LODDS model.
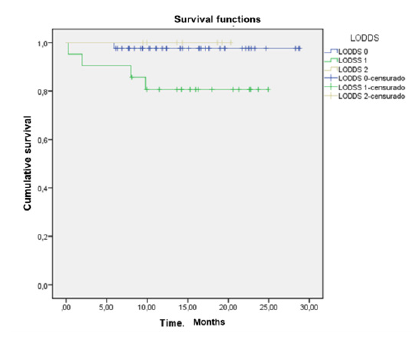
Figure 4:
Figure 5:

References
- Siegel RL, Miller KD, Jemal A (2018) Cancer statistics. CA Cancer J Clin 68(1): 7-30.
- Jessup JM, Golberg RM, Asare EA, Benson AB, Brierley JD, et al. (2017) Colon and rectum. In MBA, editor. AJCC Cancer Staging Manual. 8th edn, Springer, Chicago, USA, pp. 251-274.
- Goldstein NS (2002) Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol 26(2): 179-189.
- Sarli L, Bader G, Iusco D et al. (2005) Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer 41(2): 272-279.
- Swanson RS, Compton CC, Stewart A, Bland KI (2003) The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 10(1): 65-71.
- Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, et al. (2005) Lymph node evaluation in colorectal cancer patients: A population based study. J Natl Cancer Inst 97(3): 219-225.
- Wood P, Peirce C, Mulsow J (2016) Non-surgical factors influencing lymph node yield in colon cancer. World J Gastrointest Oncol 8((5): 466-473.
- Namm J, Ng M, Roy-Chowdhury S, Morgan JW, Lum SS, et al. (2008) Quantitating the impact of stage migration on staging accuracy in colorectal cancer. J Am Coll Surg 207(6): 882-887.
- Bilimoria KY, Bentrem DJ, Stewart AK (2008) Lymph node evaluation as a colon cancer quality measure: a national hospital report card. J Natl Cancer Inst 100(18): 1310-1317.
- Johnson PM, Porter GA, Ricciardi R, Baxter NN (2006) Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol 24(22): 3570-3575.
- Vather R, Sammour T, Kahokehr A, Connolly AB, Hill AG (2009) Lymph node evaluation and long-term survival in stage II and stage III colon cancer: A national study. Ann Surg Oncol 16(3): 585-593.
- Arslan NC, Sokmen S, Canda AE, Terzi C, Sarioglu S (2014) The prognostic impact of the log odds of positive lymph nodes in colon cancer. Colorectal Dis 16(11): O386-O392.
- Lu YJ, Lin PC, Lin CC (2013) The impact of the lymph node ratio is greater than traditional lymph node status in stage III colorectal cancer patients. World J Surg 37(8): 1927-1933.
- Scarinci A, Di Cesare T, Cavaniglia D, Neri T, Colletti M, et al. (2018) The impact of log odds of positive lymph nodes (LODDS) in colon and rectal cancer patient stratifcation: A single center analysis of 323 patients. Updates Surg 70(1): 23-31.
- Lee C, Wilkinson KH, Sheka AC, Leverson GE, Kennedy GD (2016) The log odds of positive lymph nodes stratifies and predicts survival of high-risk individuals among stage III rectal cancer patients. Oncologist 21(4): 425-432.
- Persiani R, Cananzi FC, Biondi A (2012) Log odds of positive lymph nodes in colon cancer: A meaningful ratio-based lymph node classification system. World J Surg 36(3): 667-674.
- Wang J, Hassett JM, Dayton MT, Kulaylat MN (2008) The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J Gastrointest Surg 12(10): 1790-1796.
- Occhionorelli S, Andreotti D, Vallese P, Morganti L, Lacavalla D, et al. (2018) Evaluation on prognostic efficacy of Lymph Nodes Ratio (LNR) and Log Odds of positive lymph nodes (LODDS) in complicated colon cancer: The first study in emergency surgery. World J Surg Oncol 16(1): 186.
© 2023 Edgar Fermín Yan Quiroz. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






