- Submissions

Full Text
Gastroenterology Medicine & Research
The Imaging of 68Ga-FAPI-04 PET-CT in Neuroblasto-Ma-Bearing Mice
Qiying Xiang1 and Changjing Zuo1,2*
1Department of Gastroenterology, Intestinal Microbiota Transplantation, Medical Specialties Naples Unit, Mexico
2Endoscopy Service, Oncology Hospital, National Medical Center, XXI Century, Mexican Social Security Institute, Hospital Trinidad, Mexico City, Mexico
3Department of Urologist, Chairman Medical Specialties Naples in Mexico City, Mexico
*Corresponding author: Changjing Zuo, School of Medical Imaging, Xuzhou Medical University, Xuzhou 221004, Department of Nuclear Medicine, the First Affiliated Hospital (Changhai Hospital) of Naval Medical University, Shanghai 200433, China
Submission:December 09, 2022;Published: December 21, 2022

ISSN 2637-7632Volume7 Issue2
Abstract
Early diagnosis and therapy of neuroblastoma are critical which determine the prog nosis. Nuclear medicine plays a crucial role in theranostics and new radioactive agents are exploring in neuroblastoma. 68Ga-FAPI-04 has become a valuable radiotracer to detect metabolic lesions clinically. To explore the possible application of 68Ga-FAPI-04 in neuro-blastoma, we evaluated its uptake in neuroblastomabearing mice compared with 18F-FDG and extracted the xenografts for IHC assay. The PET-CT imaging showed significant up-take of tumor both in 68Ga-FAPI-04 and 18F-FDG at 50min, and the tumor-tomuscle of 68Ga-FAPI-04 (3.29±0.62) and 18F-FDG (3.25±0.32) are similar. The tumor-to-muscle was still high at 90min. IHC assay demonstrated positive FAP expression of neuroblastoma xenografts which consisted with the PET-CT imaging. 68Ga-FAPI-04 showed high tissue contrast and prolonged uptake in neuroblastoma, and this result shows the viability of 68Ga-FAPI-04 in neuroblastoma.
Keywords:Neuroblastoma; FAPI; FDG; 68Ga; PET-CT
Introduction
Neuroblastoma is the third most common cancer of childhood, second only to leu-kemia and brain tumors [1]. Neuroblastoma arises from primitive sympathetic ganglion cells (neural crest cells), so it can arise anywhere throughout the sympathetic nervous systems. Among them, adrenal gland is the most common site (48%) followed by ex-tra-adrenal abdominal location (18%), and the remainder arise from the posterior medias-tinum, thorax, neck, pelvis and other locations. Because it has a high possibility of metas-tasis at the time of diagnosis, it accounts for nearly 15% of all childhood cancer fatalities [2]. It has been reported that children within 1 year old at diagnosis of neuroblastoma have a higher 5-year survival rate compared with those older children when received diagnosis [3]. Early diagnosis and therapy are of great significance. There are several imaging techniques to diagnosis and evaluate neuroblastoma and every method has its strengths and weaknesses. Ultrasonography (US) is the first technology to be considered when an abdominal mass is suspected in a child because it is safe without radiation and fast [4]. To further evaluate the extent of disease and assist in staging, Computed Tomography (CT) and Magnetic Resonance (MR) imaging are needed. Whole-body MR imaging show high sensitivity for detecting skeletal metastases, but the specificity remains low because it is difficult for MR to determine whether a lesion was ac-tive after treatment [5].
Nuclear medicine as a functional imaging technique has the potential to detect the tumor viability and certain molecular expression and guide the therapy regimen. MIBG is an analog of norepinephrine and can be taken up by norepinephrine transporters which expressed in up to 90% neuroblastomas [6]. Iodine 123 (123I)–labeled MIBG single photon emission computed tomography (SPECT)/CT has become the test of choice for identifying metastatic disease because of its high specificity [7]. But the use of 123I-MIBI is limited by unavailability. Fluorine 18 (18F) fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT is the most common method to evaluate tumor metabolism and activity in most tumors in clinic [8]. But the FDG response does not always correlate with the response of MIBG [9]. Octreotate labeled with 68Ga (68Ga-DOTATATE, 68Ga-DOTATOC and 68Ga-DOTANOC) are investigated currently in neuroblastoma which expressed somatostatin receptor 2 [10]. In addition, new radiotracers are exploiting to capture more meaningful information of neuroblastoma. In this study, 68Ga-FAPI-04 which targets tumor stroma was performed in neuroblastoma preclinically to preliminarily evaluate the feasibility and compare with 18F-FDG.
Materials and Methods
Preparation of radiopharmaceuticals
The precursor molecules of FAPI-04 were obtained from Shanghai Nice-labeling Bio-Technology Co., LTD. The precursors were of chemical purity above 99%. 68Ga was eluted by HCl (0.05 mol/L) from 68Ge/68Ga-generator. In a typical labeling procedure, 100μg FAPI-04 dissolved in 1mL sodium acetate (0.25mol/L) were mixed with 185MBq 68Ga eluted by 4mL HCl and reacted at 100 ℃ for 10min to get 68Ga-FAPI-04. The final product was sterile and pyrogen-free, and the radiochemical purity was > 95%. 18F-FDG was obtained from Shanghai Atomic Pharmaceutical Co., Ltd., radiochemical purity>95%.
Cells and animal models
Murine neuroblastoma cell IMR-32 was obtained from National Collection of Authenticated Cell Cultures. The cells were cultured in DMEM medium containing 10% Fetal Bovine Serum (FBS) and 1% penicillin–streptomycin. The cells grew adherent when cultured at 37 ℃, 5% CO2 and appropriate humidity. SCID female mice were obtained from Shanghai Lingchang Biotechnology Co., LTD. The animals were kept in the Animal Experiment Center of Changhai Hospital of SPF level. Then, 1 × 106 IMR-32 cells in 0.1mL mixture of PBS and Matrigel at a 1:1 ratio was inoculated subcutaneous into the right trunk of the mice. When the tumor reached an average volume of 300 – 500 mm3, experiments of imaging can be performed.
PET-CT imaging
All imaging acquisition was performed on a clinical used PETCT scanner (Bio-graph64, Siemens Healthcare, Erlangen, Germany). Before imaging, the model mice were injected with 50μL sodium pentobarbital (3%) into the abdominal cavity for anaesthesia. 3.7 MBq tracers were injected Intravenously (IV) into IMR-32 tumorbearing mice and 68Ga-FAPI-04 (n = 3) and 18F-FDG (n = 3) image scanning was performed at 50 min and 90min post-injection. For quantification of tracer uptake, 3D Regions of Interest (ROI) were drawn on the muscle and tumor.
FAP expression analysis by IHC staining
Tumor samples extracted from tumor-bearing mice were fixed with 4% paraformaldehyde. The samples were trimmed, dehydrated, embedded, sliced, stained and sealed when in good fixation in strict accordance with the instruction of pathological experiment examination. Rabbit monoclonal antibodies against FAP Rabbit anti-human FAP was used for IHC analysis. Tissue sections were scanned using a panoramic slice scanner (3DHISTECH, PANNORAMIC DESK/MIDI/250/1000). CaseViewer2.4 scanning software was used to select the target area of the slice for imaging.
Statistical analysis
Statistical analyses were performed GraphPad Prism software 8.0. Measurement data were expressed as mean ± standard deviation, and T test was used for comparison between groups. P < 0.05 was considered statistically significant.
Results
PET-CT imaging
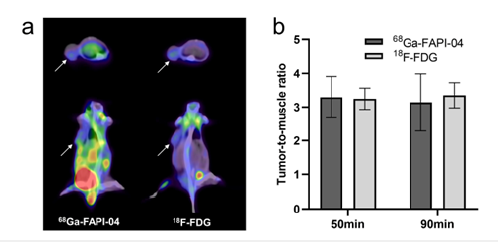
The PET-CT images showed significantly high uptake of 68Ga- FAPI-04 and 18F-FDG in IMR-32 tumors (Figure 1a & 1b) with different distribution invivo. Compared to 18F-FDG, 68Ga-FAPI-04 accumulated more in liver and gastrointestinal tract. However, the quantitative analysis demonstrated similar tumor-to-muscle-ratio based on SUVmax values of tumors in both 50min and 90min postinjection.
Figure 1: (a) PET-CT imaging (cross-sectional images and coronal images) of 68Ga-FAPI-04 and 18F-FDG in IMR-32- bearing mice at 50min post-injection. (b) The tumor-to-muscle ratio of 68Ga-FAPI-04 and 18F-FDG in 50min and 90min post-injection.
Histopathology
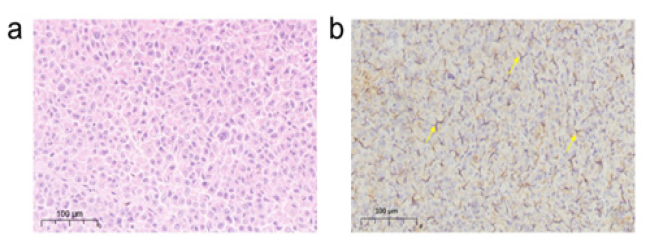
Immunohistochemical assays were performed to detect FAP level of IMR-32 xenografts. Hematoxylin and Eosin (H&E) staining revealed a good tissue morphology of IMR-32 xenografts (Figure 2a). In addition, immunohistochemical staining revealed the positive FAP expression in the IMR-32 xenografts (Figure 2b).
Figure 2: (a) Hematoxylin and eosin (H&E) staining of IMR-32 tumors (magnification, ×10). (b) Immunohistochemical staining of IMR-32 tumor xenografts using an FAP antibody (yellow arrows indicate FAP positive) (magnification, ×10).
Discussion
Fibroblast Activation Protein (FAP) is a type II transmembrane serine protease and is overexpressed on Cancer-Associated Fibroblasts (CAFs) found in the tumor stroma of various tumors [11]. Several FAP Inhibitors (FAPI) variants have been labeled with multiple radionuclides for PET and SPECT imaging and show great potential in detecting pan-cancer [12-15]. Among these radiotracers, FAPI-04 labeled with 68Ga presents remarkably high uptake and image contrast in several highly prevalent cancers through the implement on 28 different tumor entities in clinic patients [16]. Sarcoma, esophageal, breast, cholangiocarcinoma, and lung cancer patients showed the highest uptake (SU-Vmax >12), while the lowest uptake of 68Ga-FAPI-04 (SUVmax <6) was detected in pheochromocytoma, renal cell, differentiated thyroid, and gastric cancers were. 68Ga-FAPI-04 has shown excellent application in clinical diagnosis and is superior to 18F-FDG in de-tecting small metastases [17]. However, there are almost no studies of 68Ga- FAPI- 04 in neuroblastoma. In this study, we found that 68Ga-FAPI-04 PETCT imaging demonstrated the uptake in the neuroblastoma tumors which is according with the IHC result of positive-FAP expression. Although the uptake in liver and gastrointestinal tract could influence the imaging of tumor, the tumor-to-muscle demonstrated its good contrast similar to the common radiotracer 18F-FDG.
FAP-targeting radiotracers can not only assist in tumor detection, but also show potential in therapeutics. By labeling with therapeutic radionuclides 90Y, two metastatic breast cancer patients tolerated treatments very well with 90Y-FAPI-04, indicating that the treatment was safe [18]. Kuyumcu et al. [19] used lowdose 177Lu-FAPI-04 to evaluate radiation-absorbed doses to normal organs, finding that the dose to critical organs was significantly low [19]. Recently, 177Lu-FAPI2286 was reported a higher radiation dose to pancreatic cancer lesions in clinic and presented excellent therapeutic effect [20]. A series of re-searches show the promising application of radionuclide labeling-FAP variant in theranostic. However, the experience on targeted radionuclide applications is mainly restricted to a small number of disease entities, and exploration to neuroblastoma is meaningful.
Foundation
2020YPT002.
Conclusion
We constructed mouse neuroblastoma models and detected the tumor uptake successfully with 68Ga-FAPI-04 PET-CT. Compared to 18F-FDG, 68Ga-FAPI-04 showed more up-take in liver and gastrointestinal tract, but the tumor-to-muscle is similar. The result of FAP expression by IHC consisted with the PET-CT imaging.
References
- (2014) American Cancer Society. Cancer facts & figures. American Cancer Society, USA.
- Ward E, DeSantis C, Robbins A (2014) Childhood and adolescent cancer statistics. CA: A cancer journal for clinicians 64(2): 83-103.
- London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, et al. (2005) Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol 23(27): 6459-6465.
- Dumba M, Jawad N, McHugh K (2015) Neuroblastoma and nephroblastoma: A radiological review. Cancer Imaging 15(1): 1-14.
- Goo HW, Choi SH, Ghim T, Moon NH, Seo JJ (2005) Whole-body MRI of paediatric malignant tumours: Comparison with conventional oncological imaging methods. Pediatric radiology 35(8): 766-773.
- Vik TA, Pfluger T, Kadota R, Castel V, Tulchinsky M, et al. (2009) 123I‐mIBG scintigraphy in patients with known or suspected neuroblastoma: Results from a prospective multicenter trial. Pediatr Blood Cancer 52(7): 784-790.
- Sharp SE, Trout AT, Weiss BD (2016) MIBG in neuroblastoma diagnostic imaging and therapy. Radiographics 36(1): 258-278.
- Uslu L, Donig J, Link M, Rosenberg J, Quon A, et al. (2015) Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med 56(2): 274-286.
- Mueller WP, Coppenrath E, Pfluger T (2013) Nuclear medicine and multimodality imaging of pediatric neuroblastoma. Pediatr Radiol 43(4): 418-427.
- McElroy KM, Binkovitz LA, Trout AT, Czachowski MR, Seghers VJ, et al. (2020) Pediatric applications of dotatate: Early diagnostic and therapeutic experience. Pediatr Radiol 50(7): 882-897.
- Fearon DT (2014) The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res 2(3): 187-193.
- Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, et al. (2019) 68Ga-FAPI PET/CT: Biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med 60(3): 386-392.
- Giesel FL, Adeberg S, Syed M, Lindner T, Jiménez-Franco LD, et al. (2021) FAPI-74 PET/CT using either 18F-AlF or cold-kit 68Ga labeling: Biodistribution, radiation dosimetry, and tumor delineation in lung cancer patients. J Nucl Med 62(2): 201-207.
- Lindner T, Altmann A, Krämer S, Kleist C, Loktev A, et al. (2020) Design and development of 99mTc-labeled FAPI tracers for SPECT imaging and 188Re therapy. J Nucl Med 61(10): 1507-1513.
- Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, et al. (2019) Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med 60(10): 1421-1429.
- Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, et al. (2019) 68Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J Nucl Med 60(6): 801-805.
- Qiufang L, Shi S, Xu X, Yu X, Song S, et al. (2021) The superiority of [68Ga]-FAPI-04 over [18F]-FDG PET/CT in imaging metastatic esophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging 48(4): 1248-1249.
- Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, et al. (2018) Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med 59(9): 1415-1422.
- Kuyumcu S, Kovan B, Sanli Y, Buyukkaya F, Simsek DH, et al. (2021) Safety of fibroblast activation protein–targeted radionuclide therapy by a low-dose dosimetric approach using 177Lu-FAPI04. Clin Nucl Med 46(8): 641-646.
- Baum RP, Schuchardt C, Singh A, Chantadisai M, Robiller FC, et al. (2022) Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using 177Lu-FAP-2286: First-in-humans results. J Nucl Med 63(3): 415-423.
© 2022 Changjing Zuo. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






