- Submissions

Full Text
Gastroenterology Medicine & Research
The Liquor Containing Pueraria Ameliorates Hepatic Fibrosis in Alcoholic Liver Disease
Xiaobo Zhao1, Lizhi Zhu2* and Fengping Ye3*
1Yuzhe Healthcare Research Center, Tianjin 300308, China
2Department of Nuclear Medicine, Shanghai Changhai Hospital, Shanghai 200433, China
3Department of Radiology, Shanghai Changhai Hospital, Shanghai 200433, China
*Corresponding author: Lizhi Zhu, Fengping Ye, Department of Nuclear Medicine, Shanghai Changhai Hospital, Shanghai 200433, China, Department of Radiology, Shanghai Changhai Hospital, Shanghai 200433, China
Submission:December 8, 2022;Published: December 16, 2022

ISSN 2637-7632Volume7 Issue2
Abstract
Pueraria, an edible vine used extensively for numerous medical applications, contains the main flavonoid component known as puerarin. Pueraria has been used for centuries in China to counteract alcohol intoxication. In the present study, we investigated the effects of puerarin on hepatic fibrosis in SD rats induced by alcohol administration via assessing liver-related biochemical indicators, CT imaging, and pathological changes. Pathological analysis revealed that the rats given puerarin effectively alleviated the signs of hepatic fibrosis. Puerarin treatment decreased the levels of serum γ-Glutamyl Transpeptadase (GGT), Alkaline Phosphatase (ALP), Total Bilirubin (TBIL) and Direct Bilirubin (DBIL), which are indicators of hepatic cell disruption. CT image results showed that the mean CT value of liver of the pueraria plus alcohol group was significantly lower than that of the alcohol induced group. Consequently, all these results showed that puerarin could ameliorate alcohol-induced hepatic fibrosis.
Keywords:Hepatic fibrosis; Alcoholic liver disease; Pueraria; Puerarin; CT
Introduction
Hepatic fibrosis is a pathological process in which extracellular matrix accumulates and forms scar tissue due to chronic liver injury caused by various factors, including but not limited to alcoholism, vital hepatitis, and steatohepatitis [1]. Hepatic fibrosis will progress to irreversible cirrhosis and even liver cancer without timely intervention [2]. Recent studies indicate that hepatic fibrosis is reversible when the causative agent(s) is removed in the early stage [3,4]. Therefore, timely intervention before hepatic fibrosis develops into irreversible lesions is warranted. In recent years, traditional Chinese medicine has attracted much attention in the treatment of hepatic fibrosis due to its multi-component and multi-target characteristics. The traditional Chinese medication Pueraria, contains a bioactive isoflavone glucoside called puerarin (4′,7-dihydroxy-8-β-d-glucosylisoflavone), which is a C-glycoside compound and has been shown to have several anti-inflammatory and anti-fibrosis characteristics [5-7]. According to reports, puerarin has an inhibitory effect on Transforming Growth Factor (TGF)-β expression in cardiac tissues, preventing myocardial fibrosis [8]. Puerarin can alleviate kidney fibrosis in renovascular hypertensive rats [9]. Regarding the liver, puerarin exhibits hepatoprotection against CCl4-induced hepatic fibrosis in mice [10]. However, the anti-alcoholic fibrosis effect of pueraria has not been fully confirmed. This study comprehensively evaluated the effects of puerarin on alcoholic hepatic fibrosis in rats through blood biochemical indicators, pathology and imaging, and proposed a basis for puerarin to improve alcoholic fibrosis..
Methods
Male Sprague-Dawley (SD) rats (300 ± 10g) were purchased from Beijing Sibeifu Biotechnology Co., Ltd. All rats were kept at 22 ℃ with 12:12 h light-dark cycle. Animal handling and procedures were carried out in accordance with international guidelines for the use and care of laboratory animals. The experimental protocol was authorized by the local ethics committee. During the experiment, rats were divided into two treatment groups: (1) alcohol (n = 8, 42% ethanol by volume alcohol, Niulanshan Liquor); (2) pueraria plus alcohol (n = 8, 42% ethanol by volume alcohol; Yuzhe Liquor). All treatments were administered by gavage once daily. The initial dose of alcohol was 5mL/kg/d, increased at week 2 as tolerance developed to a maintenance dose of 20mL/kg/d and continued for 4 weeks at all. CT images were collected in axial, coronal and sagittal view using liver window (L: 150HU, W: 30HU). The acquired CT images were transferred to United Imaging Medical Processing Software (uWS-CT, United Imaging). The Region of Interest (ROI) was drawn on the liver and spleen, and the average CT value was measured. After CT examination, the activities of serum Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), γ-Glutamyl Transpeptadase (GGT), Alkaline Phosphatase (ALP), Total Bilirubin (TBIL) and Direct Bilirubin (DBIL) were measured using the commercial assay kits, and liver pathological changes were evaluated by Haematoxylin and Eosin (H&E) staining. Hepatic fibrosis was analyzed by Sirius Red staining according to the manufacturer’s instructions. Statistical analyses were performed using SPSS 23.0 software (SPSS Inc., USA). Data were expressed as the mean ± SD. Student’s t test was used for data comparisons, and p < 0.1 was considered as statistically significant.
Results
Effect of puerarin on liver density of CT imaging
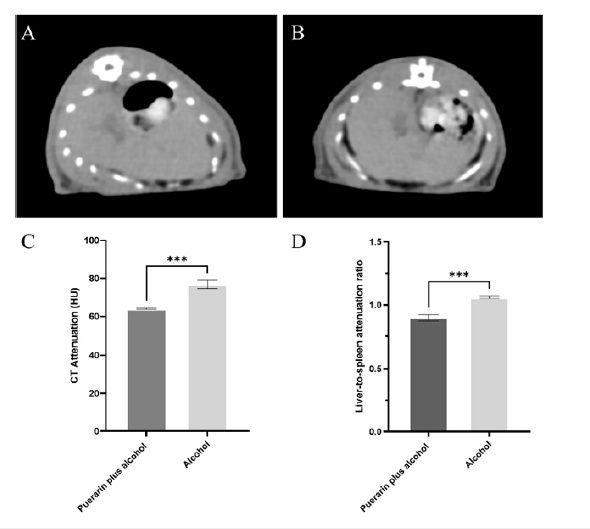
Typical CT plain scans of the two groups of rats are presented in Figure 1. CT images showed diffusely increased hepatic uptake in the two groups of rats. However, the mean CT value of liver of the pueraria plus alcohol group was significantly lower than that of the alcohol-induced group. The mean CT value of the liver in the alcohol-induced group was 76.87 ± 1.81, the mean CT value of the spleen was 72.47 ± 1.72, and the ratio of liver to spleen CT value was 1.06 ± 0.01. In contrast, the mean CT value of the liver in the pueraria plus alcohol group was 64.07 ± 0.31, the mean CT value of the spleen was 71.36 ± 1.64, and the ratio of liver to spleen CT value was 0.90 ± 0.02. There were statistically significant differences in the mean CT value of the liver and the ratio of liver to spleen CT values between the two groups of rats (p< 0.05)
Figure 1: A. Typical cross-sectional CT images of the pueraria plus alcohol. B. Alcohol groups of rats. C. The mean CT values of the liver of the two groups of rats. D. The ratio of liver to spleen CT values in the two groups of rats.
Effects of puerarin on serum ALT, AST, GGT, ALP, TBIL and DBIL
The serum ALT, AST, GGT, ALP, TBIL and DBIL levels were measured at 4 weeks after gavage with alcohol in presence of puerarin. As shown in Table 1, ALT, AST and ALP levels in the puerarin plus alcohol group were higher than those in the alcohol group [(28.59 ± 5.23) U/L vs. (26.94 ± 8.26) U/L, p = 0.70; (23.91 ± 5.63) U/L vs. (22.49 ± 7.94) U/L, p = 0.74; (230.46 ± 26.70) U/L vs. (197.73 ± 33.96 U/L), p = 0.10]. Serum GGT, TBIL and DBIL levels in the puerarin plus alcohol group were lower than those in the alcohol group [(7.73 ± 2.19) U/L vs. (8.24 ± 4.04) U/L, p = 0.80; (35.70 ± 10.19) U/L vs. (48.31 ± 9.48) U/L, p = 0.06; (4.91 ± 0.10) U/L vs. (6.10 ± 4.20) U/L, p = 0.52]. The difference between the two groups in ALT, AST, GGT, ALP and DBIL was non-significant. The difference between the two groups in TBIL was significant (p< 0.1).
Table 1:Effect of puerarin on liver related serum indexes in rats.
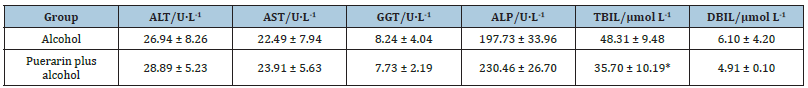
*p< 0.1
Effects of puerarin on liver pathology
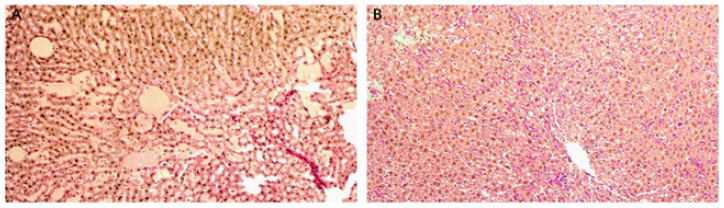
As shown in Figure 2, after the intervention of alcohol, the morphology of liver tissue cells was irregular. In detail, the hepatic cords were arranged disorderly and the structure of the liver lobules was blurred. In addition, round fat vacuoles (lipid droplets) were scattered in the liver. In the puerarin intervention group, the cell structure was complete and the hepatic cord arrangement was significantly improved. The fat vacuoles were reduced, and no obvious degeneration, necrosis and inflammatory infiltration were observed.
Figure 2: A. Sirius Red staining of liver in alcohol. B. Pueraria plus alcohol group.
Discussion
Several chronic liver diseases frequently result in hepatic fibrosis. Hepatocellular carcinoma can occur as a result of chronic fibrosis, according to abundant evidence [11]. Interrupting and/or reversing hepatic fibrosis is critical in preventing the development of hepatocellular carcinoma. However, there is no well-validated therapy for reversing liver fibrosis at this time. The potential of puerarin to alleviate alcohol-induced hepatic fibrosis was proven in this study via assessing liver-related biochemical indicators, CT imaging, and pathological changes. Chronic alcohol consumption caused considerable damage to hepatocytes, resulting in pathological elevation of the serum levels of ALT, AST, GGT, ALP, TBIL and DBIL. However, on administering puerarin, we have observed decreased levels of serum GGT, TBIL and DBIL, supporting the hypothesis that puerarin could reduce hepatic fibrosis by metabolizing total bilirubin. In addition, histological analysis showed that the rat model of alcohol-induced fibrosis was successfully established, which was characterized by marked fatty degeneration and peri hepatocyte fibrosis. Furthermore, histological analysis showed that the pathogenic characteristics of liver tissues were significantly reduced in the puerarin therapy group. CT diagnosis of hepatic fibrosis could be preferred because of its repeatability, non-invasiveness, and ability to dynamically assess the lesion. This study noninvasively assessed the effect of puerarin on fibrosis by CT imaging, which was manifested in the decrease of diffuse CT density of the liver compared with the alcohol-induced group. In conclusion, the results of this study suggest that puerarin is effective in the treatment of alcohol-induced liver fibrosis in rats. The main mechanism of this therapeutic effect may be due to its protection against liver injury by metabolizing total bilirubin. Therefore, based on our results, puerarin should be considered as a drug or an additive with potential application in the prevention and treatment of alcoholic liver fibrosis.
Conclusion
Puerarin may serve as a candidate with high potential to manage hepatofibrosis via effectively ameliorating the metabolism of total bilirubin.
References
- Ignat SR, Dinescu S, Hermenean A, Costache M (2020) Cellular interplay as a consequence of inflammatory signals leading to liver fibrosis development. Cells 9(2): 461.
- Berumen J, Baglieri J, Kisseleva T, Mekeel K (2021) Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech Dis 13(1): e1499.
- Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, et al. (1998) Mechanisms of spontaneous resolution of rat liver fibrosis. hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest 102: 538-549.
- Issa R, Williams E, Trim N, Kendall T, Arthur MJP, et al. (2001) Apoptosis of hepatic stellate cells: Involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut 48(4): 548-557.
- Zhang Z, Lam TN, Zuo Z (2013) Radix puerariae: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. The Journal of Clinical Pharmacology 53(8): 787-811.
- Zheng W, Yang M, Wu C, Su X (2019) Hydrogen peroxide through inhibiting inflammatory 9.
- Zhu X, Wang K, Zhang K, Lin X, Zhu L, Zhou F (2016) Puerarin protects human neuroblastoma sh-sy5y cells against glutamate-induced oxidative stress and mitochondrial dysfunction. Journal of Biochemical and Molecular Toxicology 30(1): 22-28.
- Chen R, Xue J, Xie M (2012) Puerarin prevents isoprenaline-induced myocardial fibrosis in mice by reduction of myocardial TGF-Β1 expression. J Nutr Biochem 23(9): 1080-1085.
- Bai S, Huang ZG, Chen L, Wang JT, Ding BP (2013) Effects of felodipine combined with Puerarin on ACE2-Ang (1-7)-mas axis in renovascular hypertensive rat. Regul Pept 184: 54-61.
- Li R, Xu L, Liang T, Li Y, Zhang S, Duan X (2013) Puerarin mediates hepatoprotection against CCl4-induced hepatic fibrosis rats through attenuation of inflammation response and amelioration of metabolic function. Food Chem Toxicol 52: 69-75.
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, et al. (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7: 6.
© 2022 Lizhi Zhu And Fengping Ye. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






