- Submissions

Full Text
Gastroenterology Medicine & Research
Lower Gastrointestinal Bleeding Exploration in 120 Ciadians Patients
Tahir Mahamat Saleh1, Ali Mahamat Moussa2*, Moustapha Mahamat Alkher1, Moussa Kally3, Mayanna Habkreo2, Assi Constant1 and Choua Ouchemi3
1Service de Medicine Interne, CHU La Renaissance, N’Djamena, Tchad
2Service de Medicine Interne et Gastroenterology, CHU La Reference National, N’Djamena, Tchad
3Service de Chirurgie Generale, CHU La Référence Nationale, N’Djamena, Tchad
*Corresponding author: Ali Mahamat Moussa, Service de Medicine Interne et Gastroenterology, CHU La Reference National, N’Djamena, Tchad
Submission:September 05, 2022;Published: September 19, 2022

ISSN 2637-7632Volume7 Issue1
Abstract
Introduction and aim: Lower Gastrointestinal (GI) bleeding is not well documented in Chad despite its frequency. The aim of this work was to study patients with lower GI bleeding, as well as the lesions attributable to this bleeding.
Patients and method: This was a retrospective, descriptive and analytical study carried out at the Internal Medicine department from January 1, 2015 to October 30, 2020. The medical records of patients who performed a complete colonoscopy for hematochezia have been included. Epidemiological, clinical, biological and endoscopic data were collected.
Results: in term of frequency lower GI bleeding constitutes 21.6% (120/555) of the reasons for requesting a colonoscopy. The mean age of patients with lower GI bleeding was 44 ± 15 years; the median of 42 years (5-78 years) and the modal class of 36-55 years (35%); the sex ratio was 4. The mean duration of the development of hematochezia was 1 year ± 2.9 years (range 3 days and 30 years); the amount of bleeding was low in 76.7% of cases (n = 92). They occurred post-fecal in 43.3% (n = 52) of cases; associated with constipation (26%; n = 31), proctalgia (20%; n = 24) and altered general status (10%; n = 12). Severe anemia was present in 4.3% (n = 4). The anoscopy, performed in 60% of patients, and was abnormal in 50.8% of cases (n = 61). Hemorrhoids (56.6%; n = 61) and colitis (27.8%; n = 30) were the most listed lesions. Patients less than 44 years of age had significantly anal fissure (p = 0.02); those over 44 years had colonic diverticula (p = 0.027) or colorectal cancer (p = 0.001). Hemorrhoids and colorectal cancer were more common in men (p = 0.033) all cancer patients had anemia (p= 0.001). Cancers (100%; n = 9), colitis (93.3%; n = 28) and anal fissures (75% n = 12) were the three main lesions attributed to hematochezia.
Conclusion:Lower GI bleeding, usually well tolerated, was frequent in gastrointestinal endoscopic exploration in young adult men without comorbidity. The cost-effectiveness of colonoscopy associated with anoscopy was high. Colorectal cancer was found in 8.3% of cases.
Keywords: Lower gastrointestinal bleeding; Etiology; Epidemiology; Clinic; Chad
Introduction
Lower GI bleeding or hematochezia is the emission of bright red blood from the anus. It usually reflects a lower digestive hemorrhage related to a lesion located downstream of the angle of Treitz. Exceptionally, the lesion may be located upstream of the Treitz angle, in case of heavy bleeding or when the intestinal transit is accelerated [1]. It is estimated that 30 to 40% of bleeding in the digestive tract originates downstream of the Treitz angle [2]. The incidence of recovery for lower GI bleeding has decreased slightly in the United States but remains an important cause of morbidity and mortality [3]. Like any GI hemorrhage, lower GI bleeding can be life-threatening due to its severity and the setting in which it occurs [4]. The management of Lower GI bleeding has evolved in recent years to include a multidisciplinary management strategy. The etiologies vary according to the age and comorbidities of the patients. Initial resuscitation and the principle of optimizing patient status prior to endoscopic evaluation are the cornerstones of clinical care [5]. Endoscopic evaluation is based on colonoscopy preceded by a proctological examination [6]. Several studies have been carried out in Africa on lower GI bleeding [7-10]. However, in Chad, although data on upper GI bleeding are available, to our knowledge very few data are available on lower GI bleeding [11]. The aim of this work was to study patients with lower GI bleeding, as well as the lesions attributable to this bleeding.
Patients and Methods
This is a retrospective, descriptive and analytical study which concerned patients received in the Internal Medicine Department of the CHU La Renaissance of N’Djamena for lower GI bleeding during the period from January 2015 to October 30, 2020. The medical records of patients explored by colonoscopy for hematochezia were included in the study. Patients with unexploitable medical records and with incomplete colonoscopy were excluded from the study. Sociodemographic, clinical data, anal margin examination data (search of anal fissure and/or fistula, hemorrhoidal prolapse), biological (hemogram) and endoscopic data: results of anuscopy with the presence of internal and/or external hemorrhoids, colonoscopy (technical conditions of realization, quality of the preparation judged subjectively according to the operator or according to the Boston score) were collected. The colonoscopy were performed under general anaesthesia (the type of anaesthesia was specified as well as the drugs used), the tolerance according to the subjective evaluation of the operator, the characteristics of the lesion (site, aspect) were collected. The lesions described in the report by the operator were grouped into colorectal polyps, colorectal cancers, diverticula, varicose veins, colitis, hemorrhoids, fissures.
The diagnosis of cancer was suspected at endoscopy and confirmed histologically. Diagnostic cost-effectiveness was defined as the percentage of colonoscopies that showed one of the lesions described above. The responsibility of the lesion as the source of the bleeding was determined by the operator’s conclusion. When there was evidence of direct or indirect bleeding from the lesion, the bleeding was attributed to it. We performed an exhaustive recruitment of all records of patients admitted for colonoscopy for hematochezia during the study period from the electronic registry of the endoscopy unit.
For the explorations, Fujinon* K056 and K057 colonoscopes were used. All examinations were performed with adult colonoscopes.
Data Analysis
The data were entered and analyzed using SSPS software version 25 (United States). First, we performed a descriptive analysis of all the variables studied. These results were presented in the form of percentages for the qualitative variables and means (with their standard deviation) for the quantitative variables. Then a bivariate analysis was performed to compare the characteristics of the lesions according to various determinants (age, sex, presence or absence of anemia, site). The comparison of percentages was done using Fisher’s exact test. A significance level of p < 0.05 was used. On the ethical level, we obtained authorization from the Faculty of Human Health Sciences (FSSH) of Ndjamena University and from the managers of the CHU la Renaissance.
Results
Table 1: Characteristics of bleeding.
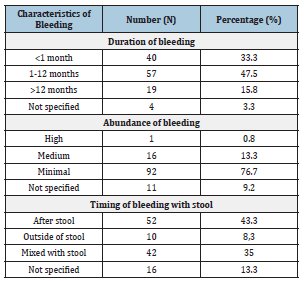
During the study period, out of 555 colonoscopies performed, 120 were for lower GI bleeding, i.e. a frequency of 21.6%. The mean age of the patients with hematochezia was 44 ±15 years; the median was 42 years (extremes: 5 -78 years) with a sex ratio M/F of 4, of which 1/3 of the patients belonged to the age group 36-55 years. The characteristics of the bleeding were evaluated (Table 1). Thus, the mean duration of evolution of hematochezia in patients studied was 1 year ± 2.9 months with extremes of 3 days and 30 years. The abundance of bleeding was minimal in the majority of cases (76.7%). The hematochezia bleeding occurred after the stool in 43.3% of cases and was associated with constipation and proctalgia respectively in 26% and 20% of cases. In terms of investigations with anuscopy and colonoscopy, anuscopy was performed in 60% of patients. It was found to be normal in 9.2% of cases, 12.5% had external hemorrhoidal disease, 20.8% internal, in 17.5% it was mixed. The majority of patients had performed colonoscopy under General Anesthesia (GA) except in 3.3% of patients. The following products were used for GA: propofol alone (7343%), propofol + fentanyl (11.7%), midazolam + fentanyl (7.5%), propofol +midazolam (2.5%) and ketamine (1.7%). The colonic preparation was performed with polyethylene glycol 4000 (PEG 4000) in all patients. The quality of the preparation was judged good by the operator in 90% of cases. Out of 120 included patients, 12 or 10% had no endoscopic lesions (strictly normal in exploration). The remaining 108, i.e. 90%, had at least one lesion. Hemorrhoids (56.5%) and colitis (27.8%) were the most common lesions. Concerning the site of the lesion, a lesion was found at the anal area in 71.3% of cases, cancers (100%), colitis (93.3) and anal fissures (75%) were the three main lesions attributed to lower GI bleeding (Table 2). In addition, patients under 44 years of age were more likely to have an anal fissure (p=0.02); those over 44 years of age were more likely to have a colonic diverticulosis (p=0.027) or cancer (p=0.001) (Table 3). In addition, hemorrhoids (p=0.033) and cancers (p=0.029) were statistically more frequent in male patients (Table 4). Finally, regarding the impact of bleeding on the blood count, anemia was significantly related to colorectal cancer.
Table 2: The various types of lesions found and the sites.
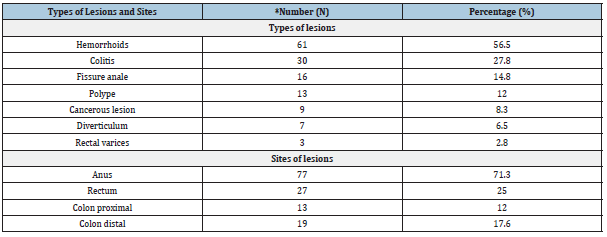
*The same patient could have several lesions.
Table 3: Relationship between age and lesion.
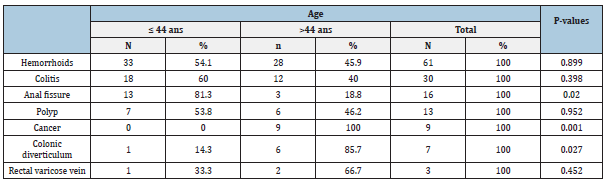
Table 4: Relationship between gender and lesion.

Discussion
We conducted a retrospective, descriptive and analytical study to investigate rectorrhagia at the CHU-la Renaissance from January 1, 2015 to October 30, 2020. We would like to point out that the retrospective nature of the work could be a source of bias on the data, especially epidemiological data. At the end of this study, we found that 21.6% of patients who underwent colonoscopy had hematochezia as a reason. This frequency is similar to that reported by Hassan et al in Morocco (22.4%) and by OKON et al in Ivory Coast (23.3%) [9,12]. A lower frequency (19.2%) was noted by Canard et al in France [13] in contrast to the study by Ankouane et al who reported a higher frequency (30%) in Cameroon [14]. This frequency varied according to the types of lower endoscopic explorations included. In Mali, a frequency of 38.8% was found when anuscopy, recto-sigmoidoscopy and colonoscopy were included [15]. Klotz et al. [16] in Gabon reported 33.5% of indications for sigmoidoscopy for rectal discharge [16]. The average age of our patients was 44 ± 15 years, close to those reported in Sub-Saharan Africa (42 to 50 years) [7,10,17]. This average age was one to two decades higher in Asia and the West respectively [18,19]. The male predominance found in our series was usual in the literature [2,20,21]. In our study, hematochezia was mainly associated with proctalgia and constipation. This preferential association was also noted by Katil et al. [15]. Deep anemia was rare but was associated in our study with the presence of colorectal cancer. This result confirms the data in the literature on the usually well tolerated nature of lower GI bleeding outside of patients at risk [3]. The protocol used in our practice for colonic preparation was judged to be good in 90% of cases in accordance with studies on the use of PEG [6,22,23]. The diagnostic efficiency was high at about 90% in this work. A similar proportion was noted by Sylvain and Beck [6,24]. Bai Y et al. [19] in a systematic review in China, observed an absence of lesion in only 4 to 7%.
In terms of etiology, hemorrhoidal disease was the most common lesion observed in this study (56.5%). It was the first cause of low endoscopic exploration [7,25,26]. It was predominantly male. In this series, hemorrhoidal disease was accompanied by anemia in less than one third of cases. Similar data have been reported by other authors [5,27,28]. The multiplicity of lesions that the same patient may have (22.5% of our patients had at least 2 lesions), suggests that in the presence of hemorrhoids, the presence of other lesions should also be sought. In the data of literature 37 to 62% of subjects had hemorrhoids associated with other lesions according to Gonver et al. [29]. Colitis was the second most common cause of hematochezia with 27.8% of cases. Paradoxically, we found a specific colitis or parasitic colitis, histology was not performed in many of the patients, nor were microbial explorations performed. This result was higher than that reported by Mbengue et al in 2009 in Dakar (17.5%) in Senegal but lower than that reported by Assi et al in 2005 in Abidjan (45%) in Ivory Coast [8,22]. The high prevalence of anal fissures could probably be explained by infectious causes, particularly bacterial and parasitic, which are frequent in our context [30]. Anal fissure is responsible for rectal bleeding in 14.8% of our patients in our series. This frequency is consistent with those observed in Sub Saharan Africa [31,32]. Colorectal polyps represent 12% of the causes of lower GI bleeding in this series. This rarity has been observed by other authors in Sub Saharan Africa, underlining their rarity in this geographical area [25,33]. However, this frequency is clearly high in the West [5,34]. The frequency of colorectal cancers found in our series is similar to those published by other authors in Ivory Coast and Mali [15,22]. As in the case of colorectal polyps, the frequency of cancers in our series was lower than those observed in Western countries [22,35,36]. The rectum was the most frequent site of colorectal cancer with 72.7% in accordance with the work of other African authors such as Mbengue et al with 88.3% [8].
The cancer was observed only in men in our series, this male predominance was also observed in several African series [7,8,37]. In the West, the tendency was more towards gender equality [33,38]. In our series, diverticula were rare in young subjects (mean age 59 years), a result that is similar to that of several other authors [22,35,39]. Colonic diverticula were found in 6.5% of cases in the study. This rarity is accepted in Sub Saharan Africa [7,8,21]. The increased incidence of diverticulosis in western countries suggests that environmental factors play a role in the pathophysiology of diverticulosis, including a low-fiber diet and lack of physical activity [40]. Furthermore, its frequency may be underestimated because we have diagnosed only hemorrhagic diverticula. The proximal colonic predominance in our study (62.5%) was also found in a study performed at the University Hospital of Cocody [22]. In the West, it is the sigmoid that is particularly affected (90%) [39]. Before the age of 44 years, anorectal lesions were predominant; they seemed to be more frequent in the colon after the age of 45 years. This anorectal lesion predominance in young subjects has been observed by other authors in Sub Saharan Africa [9,22].
Conclusion
Hematochezia is a frequent symptom in digestive endoscopy exploration. The patients were more often young adults without comorbidity. Colonoscopy is the examination of choice in the exploration of rectal bleeding, its cost effectiveness was very high. Hemorrhoidal disease and colitis were the most frequent lesions in young people, while cancers and diverticula were the prerogative of older subjects. Colorectal cancer, colitis and anal fissure were the main lesions attributed to rectal bleeding.
References
- Lesur G, Grigoriu I (2008) Lower gastrointestinal bleeding. EMC (Elsevier Masson SAS, Paris), Gastroenterology 9: 6-11.
- Peery AF, Crockett SD, Barritt AS (2015) burden of gastrointestinal, liver, and pancreatic Disease in the United States. Gastroenterology 149(7): 1731-1741.
- Almadi M, Barkun A (2018) Patient presentation, risk stratification, and initial management in acute lower gastrointestinal bleeding. Gastrointest Endosc Clin N Am 28(3): 363-377.
- Eckmann DJ, Victor G, Loftus CG (2018) A rational approach to the patient with hematochezia. Curr Opin Gastroenterol 34(1): 38-45.
- Strate LL, Gralnek IM (2016) American college of gastroenterology clinical guideline: Management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol 111(4): 459-474.
- Beck KR, Amandeep KS (2018) Colonoscopy in acute lower gastrointestinal bleeding diagnosis, timing, and bowel preparation. Gastrointest Endosc Clin N Am 28(3): 379-390.
- Drissa K, Traoré LI, Sogoba G, Sangare S, Diallo B, et al. (2020) Etiologies of rectorragies in adults in digestive endoscopy centers in Kayes (Mali). Health Sci Dis 21(9): 63-66.
- Mbengue M, Dia D, Diouf ML (2009) Contribution of colonoscopy in the diagnosis of rectal bleeding in Dakar (Senegal). Med Too 69(3): 286-288.
- Okon JB, N'dri N, Toth'o A, Assi C, Diakité M, et al. (2012) Diagnosis of rectal bleeding at the University Hospital of Cocody in Abidjan. Tropical Med and Health 22(4): 398-400.
- Djibril AM, M'ba KB, Bagny A, Kaaga L, Redah D (2009) Etiological aspects of rectal bleeding in adults in Africa: About 85 cases collected over 12 years at the CHU-Lome Campus. Mali Med 3: 40-42.
- Moussa AM, Choua O, Jean BK, Tahir MS, Assi C, et al. (2018) Clinical, etiological and prognostic profile of upper gastrointestinal bleeding in N'Djamena (Chad). Health Sci Dis 19(1): 65-68.
- Hassan GM, Kabbaj N, Amrani L, Serraj I, Guedira MM (2008) The Moroccan experience with colonoscopy: A review of 1157 cases. Arab J Gastroenterol 9: 82-84.
- Canard JM, Debette-Gratien M, Dumas R (2005) A prospective national study on colonoscopy and sigmoidoscopy in 2000 in France. Gastroenterol Clin Biol 29(1): 17-22.
- Ankouane AF, Kowo M, Ngononga B, Djapa R, Tagni-Sartre M, et al. (2013) Indications, results and yield of colonoscopy in an unfavorable economic environment: The case of Cameroon. health Sci Dis 14: 1-4.
- Katile D, Dicko MY, Conde A, Malle O, Sangare D, et al. (2019) The anorectal pathology in Kayes, Mali. Health Sci dis 20: 113‐
- Klotz F, Moussavou J, Walter P (2002) Management and diagnosis of colorectal cancers. Retrospective study carried out over 12 months in private practice. Gastroenterol Clin Biol 24: 72‑
- Diarra M, Konaté A, Kaya SA, Sangaré D (2015) Internal hemorrhoidal disease at the digestive endoscopy center of the CHU Gabriel Touré in Bamako. Mali Med 30(3): 38-41.
- Oakland K, Kothiwale SK, Forehand T, Jackson E, Bucknall C, et al. (2020) External validation of the Oakland score to assess safe hospital discharge among adult patients with acute lower gastrointestinal bleeding in the US. JAMA Netw Open 3(7): 209-630.
- Bai Y, Peng J, Gao J, Zou DW, Li ZS (2011) Epidemiology of lower gastrointestinal bleeding in China: Single-center series and systematic analysis of Chinese literature with 53951 patients. J of Gastroenterology and Hepatology 26(4): 678-682.
- Shah AR, Jala V, Arshad H, Bilal M (2018) Evaluation and management of lower gastrointestinal bleeding. DIS Mon 64(7): 321‑3
- Bougouma A, Giungane NA, Sombie RA (2012) Anorectal pathology in a hospital setting in Ouagadougou (endoscopic approach): Epidemiological and diagnostic aspects. Med Afr Noire 5: 87-94.
- Assi C, Louhoues, Kouakou MJ, Toth'o TF (2005) Some epidemiological aspects of rectal bleeding in black Africans. Med Afr black 53: 315-319.
- Kok K, Kum C, Goh P (1998) Colonoscopic evaluation of severe hematochezia in an oriental population. Verlag kg 30: 675‑
- Silvain C, Borderie C, Ripault MP, Beauchant M (1998) Digestive bleeding. Encycl Med Chir. Gastroenterology, pp. 1-12.
- Bagny A, Lawson-Ananissoh LM, Bouglouga O, Kaaga LY (2017) The anorectal pathology at the CHU-campus of Lomé (Togo). European Scientific Journal 13(3): 4.
- Dia D, Diouf D, Mbengue M, Bassene M, Fall S (2010) Anorectal pathologies in Dakar, analysis of 2016 proctological examinations. Med Afr Noire 57: 241-244.
- Aoki T, Hirata Y, Yamada A, Koike K (2019) Initial management for acute lower gastrointestinal bleeding. World J Gastroenterol 25(1): 69- 84.
- Jensen DM, Machicado GA (1988) Diagnosis and treatment of severe hematochezia: the role of urgent colonoscopy after purge. Gastroenterology 95(6): 1569-1574.
- Gonvers J, Bosset V, Froehlich F, Dubois R (1999) Appropriateness of colonoscopy: Hematochezia. Endoscopy 31(8): 631-636.
- Paul J (2020) Colonoscopic finding of patients with lower gastrointestinal bleeding at different age group in eastern part of India - an observational study. Prague Med Report 121(1): 25-34.
- Keita CO (2011) Primary anal fissures operated on in the general surgery department [thesis]. CHU-Gabriel Touré, Bamako, Mali, pp. 112.
- Tade AO, Salami BA, Musa AA, Adeniji AO (2004) Anal complaints in Nigerians attending Olabisi Onabanjo University Teaching Hospital (OOUTH), Sagamu. Niger Postgrad Med J 11(3): 218‑
- Yassibanda S, Ignaleamoko A, Mpesso P, Bobossi GS, Boua N, et al. (2004) The anorectal pathology in Bangui in Central African Republic. Mali Med 19(2): 12-14.
- Lisa L (2005) Lower gastrointestinal bleeding: Epidemiology and diagnosis. Gastroenterol Clin North Am 34(4): 643-664.
- Ghassemi KA, Jensen DM (2013) Lower GI bleeding: Epidemiology and management. Curr Gastroenterol Rep 15(7): 333.
- Matilon Y (2001) Place of virtual colonoscopy in colorectal cancer screening. In: EMC, Gastroenterology, pp. 99‑
- Dakub J, Kumoji R, Naaeder S, Cleeg-Lamptey J (2008) Endoscopic evaluation of the colorectum in patients presenting with haematozia at Korle-Bu teaching hospital Accra. Ghana Med J 42(1): 33-37.
- Boyle P, Langman JS (2000) Epidemiology -ABC of colorectal cancer. Br Med j 321(7264): 805-808.
- Floch MH, Bina I (2006) The natural history of diverticulitis: Fact ant theory. J Clin Gastroenterol 38(5 Suppl1): S2-S7.
- Chaudhary S, Khatri P, Dhakal PR, Shahi A, Jaiswall NK, et al. (2019) Clinical profile and colonoscopic findings in patients presented with lower gastrointestinal bleeding. IOSR Journal of Dental and Medical Sciences 18: 50-55.
© 2022 Ali Mahamat Moussa. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






