- Submissions

Full Text
Gastroenterology Medicine & Research
Effectiveness of Acupuncture for Recovery of Bowel Function in Patients After General Anesthesia: A Systematic Review and Meta- Analysis
Juan-Juan Zhang1*, Kai-Li Deng2, Xiao-Lei Cai3 and Li-Xing Zhuang1
1Guanzhou University of Chinese Medicine, China
2Department of Epidemiology, School of Public Health, Sun Yat-sen University, China
3Guangdong provincial people’s hospital, China
*Corresponding author: Juan-Juan Zhang, Guanzhou University of Chinese Medicine, No12, Jichang Road, Baiyun District, Guangzhou, Guangzhou 510405, Guangdong, China
Submission:June 01, 2022;Published: June 15, 2022

ISSN 2637-7632Volume7 Issue1
Abstract
The aim of this study was to evaluate the effects of Electro-Acupuncture (EA) and Transcutaneous Electrical Acupoint Stimulation (TEAS) for narrowing the duration of Postoperative Ileus (POI) and enhancing bowel function after general anesthesia.
Methods: A systematic search of electronic databases included PubMed, EMBASE, Ovid, Web of Science for studies published from 2010 until July 2021 was carried out. Randomized Controlled Trials (RCTs) involving the use of acupuncture for POI and bowel function in patients were identified. Outcomes in every work were collected and combined for determining Standardized Mean Difference (SMD) and Risk Ratio (RR). We also utilized DerSimonian and Laird approach for computing the combined SMD estimates and the respective 95% Confidence Intervals (CIs). Additionally, we conducted subgroup analysis according to intervention timing and intervention frequency.
Results: The present work enrolled 27 RCTs involving 2053 participants. Acupuncture achieved higher efficacy of releasing postoperative ileus, together with shorter time-to-first flatus/bowel sound/ defecation, shorter Length Of Stay (LOS), and greater efficacy of Postoperative Ileus relative to controls (sham acupuncture, non-acupuncture, or additional active treatments). No difference was found between the efficacy of EA and TEAS. Difference in efficacy was not significant between different type of intervention timing and intervention frequency, as revealed by subgroup analysis.
Conclusion:Electro-acupuncture and transcutaneous electrical acupoint stimulation both showed large effect size on improving postoperative gut function. More convincing data are warranted.
Keywords: Electro-acupuncture; Transcutaneous electrical acupoint stimulation; Postoperative ileus; Meta-analysis
Abbreviations: EA: Electro-Acupuncture; TEAS: Transcutaneous Electrical Acupoint Stimulation; POI: Postoperative Ileus; RCTS: Randomized Controlled Trials; SMD: Standard Mean Difference; COX: Cyclooxygenase; CI: Confidence Interval
Introduction
Bowel dysfunction is tightly associated with poor life quality after surgery, and it represents a common postoperative complication [1,2]. Bowel dysfunction is mainly caused by anesthetics, intraoperative traction and trauma, postoperative water and fasting, bed rest, and the use of analgesic drugs. Postoperative Ileus (POI) refers to transient bowel movement dysfunction that last for 3-5 days, and it can occur following diverse major surgeries [3-5]. Clinically, POI is manifested as abdominal pain, delayed flatus or stool passage, vomiting and nausea [6]. Usually, bowel functional recovery is frequently used as the POI endpoint, while treatment for POI mainly focuses on reducing time-to-first flatus/ defecation [7]. POI may lead to patient discomfort, longer Length of Stay (LOS), longer functional recovery as well as higher medical expenses [8]. The POI incidence is reported to be 10-30% [9,10], besides, it is reported that POI contributes 1.5 million dollars to economic burden annually.
Acupuncture is one of the Traditional Chinese Medicine (TCM) treatments to manage different disorders, and it has aroused more and more attention of patients and clinical practitioners globally. It is the non-pharmacological approach usually adopted to manage different Gastrointestinal (GI) diseases. According to the preclinical animal research results, acupuncture activates vagus nerve for improving the transport activity of GI tract and thereby promoting POI recovery [11]. Additionally, as reported in one work, acupuncture relaxes GI smooth muscle to modulate vasoactive intestinal peptide (VIP) contents while improving GI movement [12,13]. Moreover, acupuncture is identified to be an effective approach to restore GI barrier function [14], while Electroacupuncture (EA) pretreatment increases the levels of cyclooxygenase (COX)-1/2 and decreases gastric acidity to reduce ulcer severity caused by acetylsalicylic acid. As reported by Fang and colleagues [11], acupuncture facilitates to mitigate functional dyspepsia symptoms, and normalize braingut axis function by maintaining GI homeostasis. Currently, acupuncture-associated techniques are Transcutaneous Electrical Acupoint Stimulation (TEAS), Electronic Acupoint Stimulation (EAS), and manual acupuncture. Of them, EA refers to acupuncture after modification, which is conducted to stimulate certain specific acupoints by acceptable currents based on conventional acupuncture. On specific body line, different acupoints can be found. EA on different acupoints achieves diverse efficacy in GO diseases. Typically, Transcutaneous EA (TEA) has been recently formulated as a non-invasive intervention that requires no needle insertion. It is associated with the merits of portability and self-administration at home by patients. Both TEAS and EAS are popular among acupuncturists since they can accurately control the stimulation variables and facilitate quantitative study. Therefore, these two approaches were applied in the present work. As far as we know, meta-analysis regarding this aspect is unavailable so far. Therefore, the present systematic review and meta-analysis was conducted for the comprehensive assessment of whether acupuncture and TEAS were effective on avoiding POI while improving postoperative intestinal functions.
Materials and Methods
The present work was conducted following guidelines of Preferred Reporting Items for Systematic Reviews and Meta- Analyzes (http://links.lww.com/MENO/A579, see Supplemental Digital Content 1). Our study protocol was registered at PROSPERO registry (CRD42021283826).
Literature search and study selection
We searched electronic databases including EMBASE, PubMed, Web of Science and Ovid to identify related studies that examined how EA affected POI from inception to July 31st, 2019, by using search terms associated with EA (such as “transcutaneous electrical acupoint stimulation” OR “acupuncture” OR “electrical acupuncture” OR “TEAS”) as well as those associated with intestinal function after surgery (such as “intestinal inflation” OR “or gastrointestinal function” OR “postoperative intestinal function” OR “bowel function”). No restriction was set regarding age, sex, surgery type or date. Meanwhile, possibly related works were searched by reading titles and abstracts with the above teams. Moreover, we manually retrieved the reference lists in related studies to avoid missing any relevant article. In this work, 2 reviewers were responsible for identifying whether the studies were eligible for further analysis based on the inclusion criteria below: (1) randomized controlled trials (RCTs); (2) EAS being the experimental intervention measure (where electrical stimulation was conducted in this patient population at specific acupoints using the electrical stimulator via the electrode tabs. Accordingly, corresponding mode, intensity and frequency of the electrical stimulator were set), whereas placebo being sham EAS protocol; and (3) studies that assessed whether EAS was effective on bowel functional recovery after surgery following general anesthesia. The study exclusion criteria were as follows, (1) case reports, comments, letters, crossover studies, review articles, editorials, retrospective studies or meta-analysis; (2) animal experiments; (3) works with unavailable or insufficient data. Any disagreement between them was solved by negotiation or the opinion of corresponding author.
Data extraction and quality assessment
Data, including first author, country, publication year, surgery type, sample size, EAS intervention details and interested outcomes were extracted by 2 reviewers. The authors were contacted for studies with unavailable or inadequate data. The interested outcomes were LOS and time-to-first flatus/defecation/ bowel sound. We adopted Cochrane collaboration risk-of-bias approach to assess study quality and risks were assessed as high, low or unclear [15]. Two researchers were responsible for assessing those enrolled articles from aspects of blinding implementation, allocation concealment, method to generate random sequences, selection bias, or additional bias sources. Any discrepancy between them was settled via negotiation or the opinion from a third researcher.
Statistical analysis
We conducted meta-analysis on eligible articles using the random-effects model for determining how EAS affected intestinal functional recovery postoperatively. In addition, Standardized Mean Difference (SMD) together with the related 95% Confidence Intervals (CIs) was adopted for presenting EAS’s effect size on intestinal functional recovery. Typically, SMDs being ≤0.2, 0.5, 0.8 stood for small, moderate, and great effects, separately. In addition, we applied chi-square test to assess heterogeneities in effect sizes among different articles, whereas I2 statistic was employed to evaluate inconsistency. Further, we carried out subgroup analysis for detecting whether different factors affected the pooled effect size, including acupuncture types (i.e., EA and TEAS), treatment duration (i.e., <7 days and >7 days), and surgery type (i.e., abdominal and non- abdominal). At the same time, we conducted sensitivity analysis by eliminating the work with high bias risk according to study quality evaluation, so as to detect whether this work affected the pooled effect size. We later analyzed the difference of 2 estimated effect sizes. Publication bias was tested by Egger’s test. Effect of potential bias was corrected by Duval and Tweedie’s trim and fill test. This work adopted STATA 15.0 software for data analysis. P<0.05 stood for statistical significance.
Results and Discussion
Search results
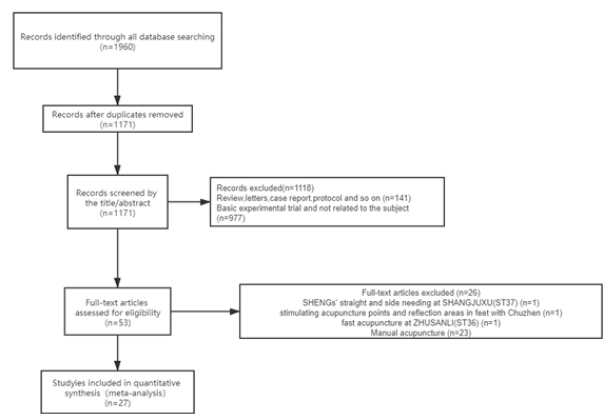
This study discovered altogether 1960 works at first. Then, we eliminated 789 works due to duplicates. Later, we screened titles and abstracts and eliminated another 1118 articles due to case reports, nonclinical trials, protocols, reviews, and irrelevance. Among those rest 53 works, we finally selected 27 after full-text screening. Figure 1 displays the study selection flowchart [16-20].
Figure 1: PRISMA flow diagram of studies included in the review.
Characteristics of included studies
Table 1 lists enrolled study information. Each of our enrolled article was RCT published between 2006 and 2020. 27 trials were conducted in China (Table 1).
Table 1: Characteristics of included clinical EA or TEAS trials of POI treatment.
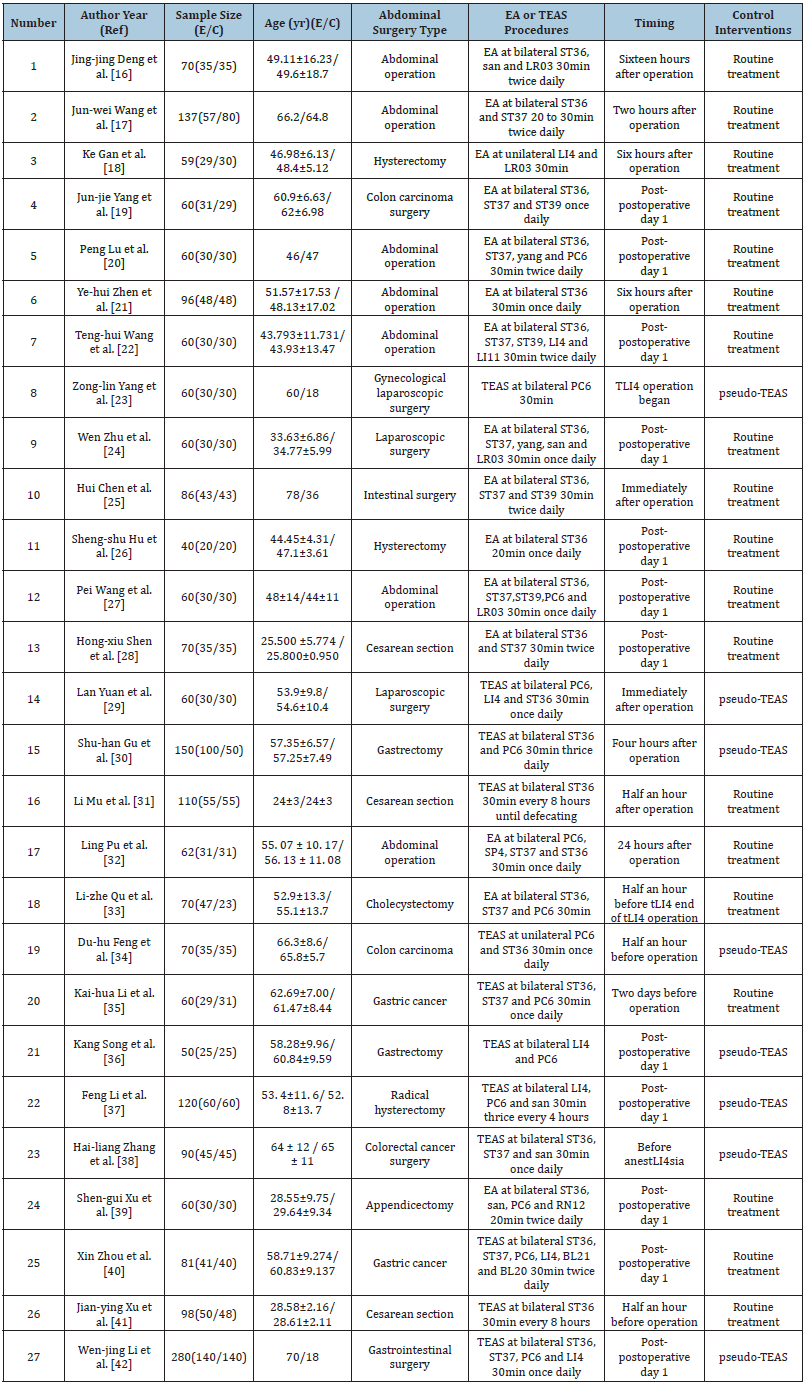
Notes: E: Experimental Group; C: Control Group; EA: Electro-Acupuncture; TEAS: Transcutaneous Electrical Acupoint Stimulation fat Mass.
Participants:Among those 27 RCTs enrolled, 2053 cases with an age of 15-79 years were recruited, and the sample sizes were 40-140. Of these cases, 945 underwent acupuncture, whereas 1108 received control treatment [21-25].
Interventions:EA was used in 15 trials, and TEAS was used in 12 trials. The intervention initiation time was reported in each RCT. Typically, ST36 (n=25) and ST37 (n=14) were two acupoints frequently adopted. The overall course of treatment was 1-10 days, while the intervention dose was 20-30 min [26-30].
Outcome measures:In a majority of RCTs, time-to-first flatus/ defecation/bowel sound were selected to be primary outcome; meanwhile, LOS (n=10) and the efficacy of promoting POI (n=7) were the frequently adopted secondary outcomes [31-35].
Risk of bias and level of evidence
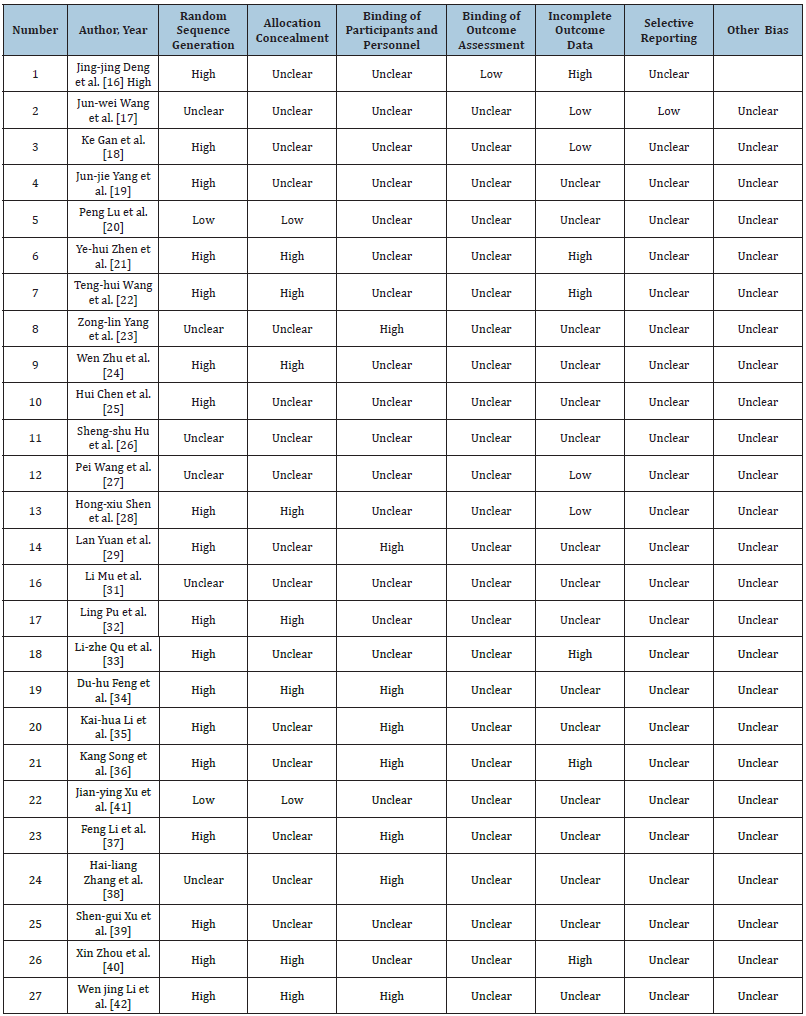
Of 27 RCTs, 19 provided details about the method to generate random sequences, while just 2 did not provide any information on this aspect. In addition, there were 8 RCTs describing details regarding allocation concealment [36-42]. Among the 27 RCTs, 8 conducted rigid study design to achieve double blinding, while the remaining 19 RCTs did not provide sufficient information regarding participant blinding or personnel. None of our enrolled RCTs provided information about outcome assessment blinding. A majority of our enrolled RCTs had low risk of bias in reporting and attrition. Table 2 summarizes the evidenced obtained.
Table 2:Quality assessment of the twenty-seven included studies with low, high, or unclear risk of bias..
Meta-analysis results
The random-effects model was utilized for data combination to compare EAS with control and TEAS with control.
Efficacy of acupuncture therapy:
Time-to-first flatus
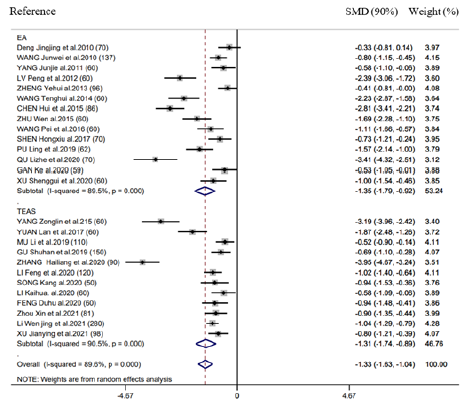
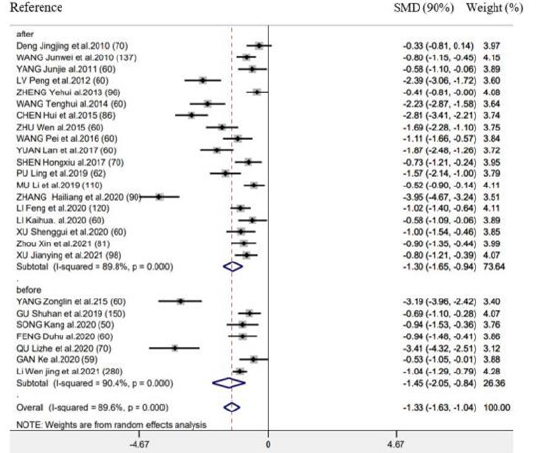
There were 26 RCTs providing information of time-to-first flatus. As revealed by combined results, EAS (EA as well as TEAS) significantly benefited this outcome (SMD = -1.33h, 95% CI: -1.63 to -1.04, 𝑃< 0.001; 𝐼2 =89.6%) (Figure 2). We also performed subgroup analysis for exploring the feasibility to explain heterogeneities via diverse acupuncture therapeutic modalities. As a result, there is no significant difference between EA (SMD = -1.35h, 95% CI: -1.79 to -0.92, 𝑃< 0.001) and TEAS (SMD = -1.31h, 95% CI: -1.74 to -0.85, 𝑃< 0.001) in association with time-to-first flatus (Figure 2). This study carried out subgroup analysis stratified by control group, Intervention timing (e.g., Before surgery and after surgery), and Intervention frequency (e.g., ≤1 time/day and ≥2 time/day). As a result, heterogeneities were not settled, even though the overall conclusion did not exhibit any difference (Supplementary Figure 1 & 2).
Figure 2: Forest plot of time-to-first flatus in EA/TEA vs control.
Supplementary Figure 1: Forest plot of acupuncture treatment versus control group on the time to first flatus: Intervention frequency (1 ≤1 time/day; 2 ≥2 time/day).
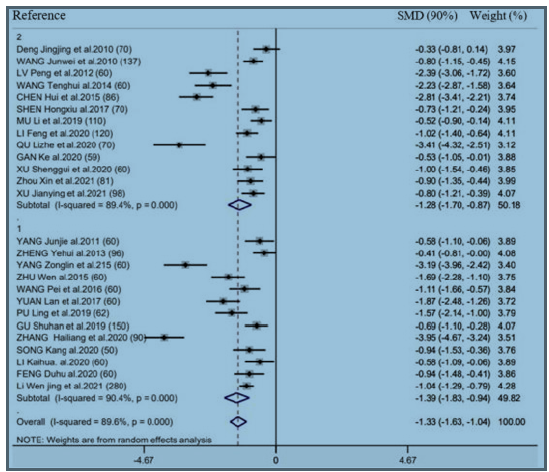
Supplementary Figure 2: Forest plot of acupuncture treatment versus control group on the time to first flatus: Intervention timing (before surgery or after surgery).
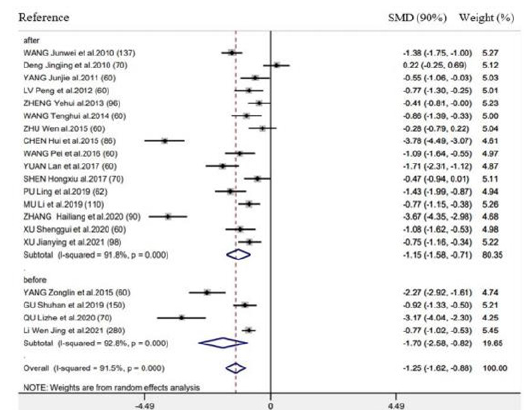
Time-to-first bowel sounds
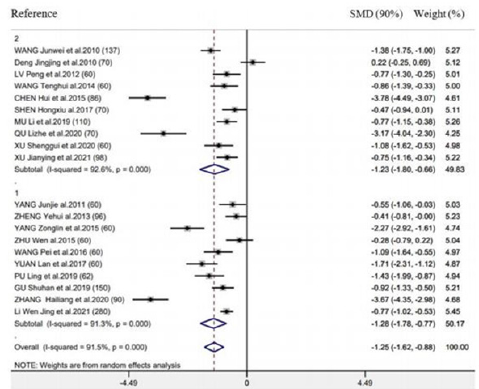
There were 20 RCTs mentioning the above outcome. As revealed by results of meta-analysis, this outcome markedly decreased in groups compared with control (SMD = -1.25h, 95% CI: -1.62 to -0.88, 𝑃< 0.001; 𝐼2 =91.5%) (Figure 3). We also performed subgroup analysis for exploring the feasibility of explaining heterogeneity via diverse acupuncture therapy modalities. As a result, there is no significant difference between EA (SMD = -1.12h, 95% CI: -1.60 to -0.63, 𝑃< 0.001) and TEAS (SMD = -1.50h, 95% CI: -1.62 to -0.88, 𝑃< 0.001) in association with this outcome (Figure 3). As suggested by subgroup analysis stratified by control group, Intervention timing (e.g., Before surgery and after surgery), and Intervention frequency (e.g., ≤1 time/day and ≥2 time/day), heterogeneities were not settled, even though the overall conclusion did not exhibit any difference (Supplementary Figure 3 & 4). Besides, subgroup analysis did not explain the possible heterogeneity source.
Figure 3: Forest plot of time-to-first bowel sound in EA/TEA vs control.
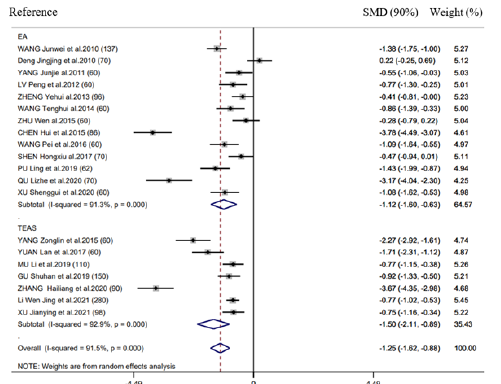
Supplementary Figure 3: Forest plot of acupuncture treatment versus control group on the time to first bowel sound: Intervention frequency (1 ≤ 1 time/day; 2 ≥ 2 time/day).
Supplementary Figure 4: Forest plot of acupuncture treatment versus control group on the time to first bowel sound: Intervention timing (before surgery or after surgery).
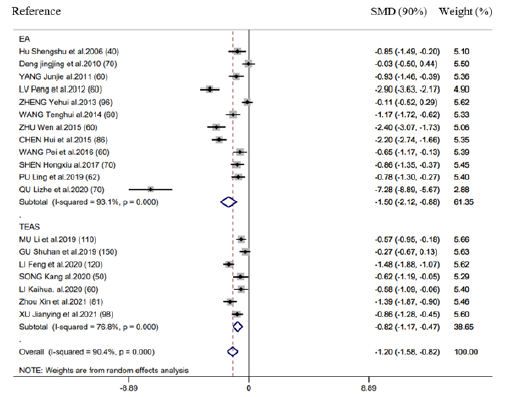
Time-to-first defecation
There were 19 RCTs reporting the above outcome. According to combined analysis, acupuncture therapy achieved the decreased value of this outcome (SMD= -1.20h, 95% CI: -1.58 to -0.82, 𝑃< 0.001; 𝐼2=90.4%) (Figure 4). Based on subgroup analysis stratified by diverse acupuncture therapeutic modalities, there is no significant difference between EA (SMD= -1.50h, 95% CI: -2.12 to -0.88, 𝑃< 0.001) and TEAS (SMD= -0.82h, 95%CI: -1.17 to -0.47, 𝑃< 0.001) in association with time-to-first defecation (Figure 4). According to subgroup analysis stratified by control group, Intervention timing (e.g., Before surgery and after surgery), and Intervention frequency (e.g., ≤1 time/day and ≥2 time/day), heterogeneities were not settled, even though the overall conclusion did not exhibit any difference (Supplementary Figure 5 & 6).
Figure 4:Forest plot of time-to-first defecation in EA/TEA vs control.
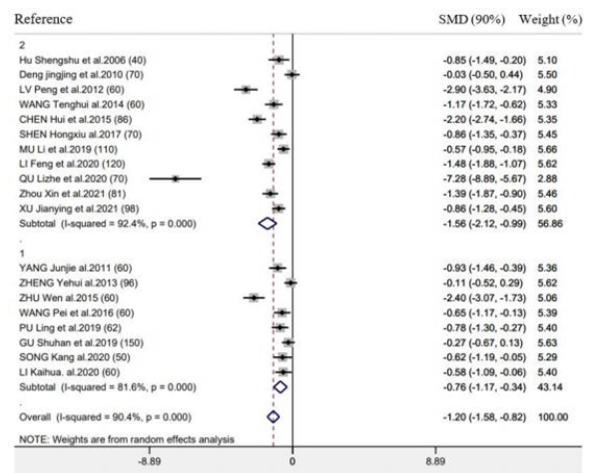
Supplementary Figure 5:Forest plot of acupuncture treatment versus control group on the time to first defecation: Intervention frequency (1 ≤ 1 time/day; 2 ≥ 2 time/day).
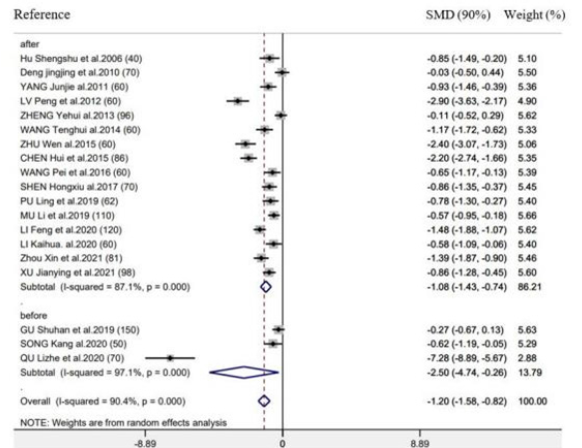
Supplementary Figure 6:Forest plot of acupuncture treatment versus control group on the time to first defecation: Intervention timing (before surgery or after surgery).
Efficacy of postoperative ileus and length of hospital stay
There were 10 RCTs mentioning the efficacy in POI, whereas 7 RCTs reporting LOS. EAS performed well in decreasing LOS (SMD = -0.52d, 95% CI: -0.81 to -0.22, 𝑃< 0.001; 𝐼2 =79.2%) (Figure 5) relative to control group, however, it did not exhibit any significant effect on decreasing the POI incidence (RR = 0.90, 95% CI: 0.78 to 1.05, 𝑃 = 0.99; 𝐼2 =0.0%) (Figure 6). Further, according to subgroup analysis, EA TEAS (SMD = -0.44h, 95%CI: -0.71 to -0.17, 𝑃< 0.001) and (SMD = -0.94h, 95% CI: -2.50 to -0.61, 𝑃< 0.001) achieved similar effect on LOS (Figure 5).
Figure 5:Forest plot of efficacy of postoperative Ileus in EA/TEA vs control.
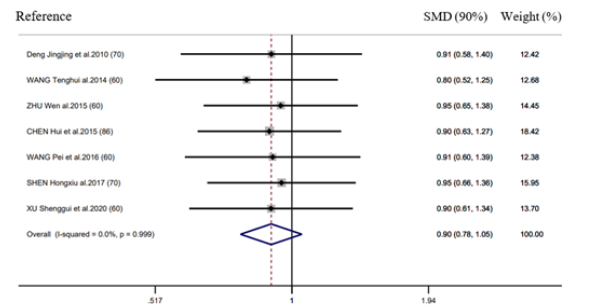
Figure 6:Forest plot of length of hospital stay in EA/TEA vs control.
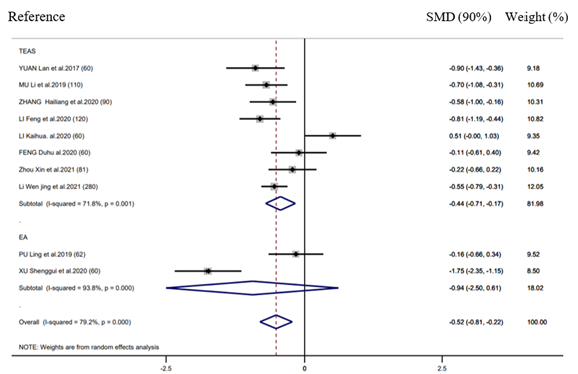
Sensitivity analysis
We conducted sensitivity analysis through eliminating an individual RCT each time to see whether this RCT affected the pooled risk estimate. As a result, eliminating one individual RCT did not make any difference to time-to-first flatus/defecation/ bowel sounds, efficacy in POI and LOS. In addition, the pooled SMD per alteration of time-to-first flatus/defecation was between -1.37 (95% CI: -1.68 to -1.07) and -1.22 (95% CI: -1.49 to -0.96) and between -1.26 (95% CI: -1.65 to -0.88) and -1.01 (95% CI: 1.33 to -0.69), separately. Those enrolled RCTs were carefully reviewed, and it was concluded that the heterogeneities did not affect acupuncture therapy’s combined effect size.
Publication bias
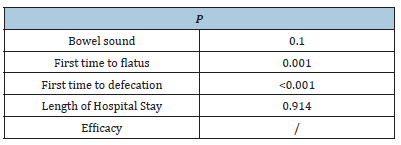
As suggested by Egger’s test, there was a possible publication bias in the relation between EAS and time-to-first flatus/defecation (P<0.050), rather than other outcomes (P>0.050, Supplementary Table 1). Afterwards, results were corrected by Duval and Tweedie’s trim and fill test. We obtained identical relations when the potential missing articles (n=3) were corrected, suggesting no influence of unpublished studies on interpreting the current observations.
Supplementary Table 1: Assessment of potential publication bias by Egger’s test..
Discussion
Acupuncture is applied in China to treat different GI diseases for a history of more than 2000 years. EA represents the modern therapy that utilizes electric currents based on conventional acupuncture to stimulate acupoints, whereas TEAS stimulates certain acupoints with no need of needles through the cutaneous electrodes. Both TEAS and EA have been increasingly popular because of their fast GI functional recovery postoperatively. The present systemic review came to the conclusion of the safety and efficacy of TEAS and EA in treating postoperative POI. Our meta-analysis findings revealed that applying TEAS and EA led to decreased time-tofirst flatus/defecation/bowel sound, reduced POI incidence and short LOS. As far as we know, the present meta-analysis aimed to examine whether TEAS and EA were effective on POI. Due to the heterogeneities in study features, this work conducted subgroup analysis stratified by diverse acupuncture types, intervention frequency and timing. As a result, EA/TEAS promoted GI functional recovery among cases receiving surgical treatment, which was significant in clinical practice. There was no side effect reported in any of those 27 RCTs. Generally, our results indicated that TEAS and EA were safe during the surgical procedure.
Nonetheless, the mechanism related to TEAS and EA remains unclear. The following reasons may be responsible for this. First, Acupuncture can stimulate somatosensory neurons, directly activate different nuclei of the central nervous system, and regulate the imbalance of sympathetic, parasympathetic, and vagus nerve activity, thereby regulating the activity of internal organs [43]. Second, as revealed by relevant articles, acupuncture at specific acupoints modulates immune cells in the body, reduce mucosal damage, and at the same time reduce postoperative inflammation and inhibit angiogenesis [44]. In addition, acupuncture can also regulate brain-gut peptides, regulate gastrointestinal physiology, relax gastrointestinal smooth muscle, and improve gastrointestinal motility. Acupuncture can also improve the microcirculation blood flow of the intestinal tract [45]. Notably, ST36 (92.6%, 25/27), ST37 (51.9%, 14/27), ST39 (22.2%, 6/27), PC6 (51.9%, 14/27) and SP6 (29.6%, 8/27) were acupoints frequently adopted. According to TCM principles, all the above acupoints are located in the “Stomach Meridian” within the surrounding lateral crural area. ST36, also called Zusanli can activate the cholinergic anti-inflammatory mechanism, reduce postoperative local inflammation, promote intestinal motility, reduce the concentration of VIP, and regulate the concentration of MTL, growth hormone and GAS, and enhance gastrointestinal motility [46,47]. ST37, also called Shangjuxu can relieve visceral pain, reduce the distribution of SP in the gastrointestinal tract, and increase the distribution of VIP [48,49]. ST39, also called Xiajuxu can significantly increase bowel motility, and PC6, also called Neiguan can inhibit gastric juice secretion and regulate gastrointestinal motility. And SP6, also called Sanyinjiao can enhance the peristalsis of the lower part of the colon and rectum, strengthen intestinal function, increase the number of white blood cells, and increase the phagocytic index of bacteria. The enrolled RCTs employed EA/TEAS at similar time points in the recovery process. EAS was most commonly conducted at 1 day after surgery (20/27), while EA/TEAS was usually conducted in 24 h preoperatively (n=7). EA before and after surgery were good for POI treatment. As suggested by an animal study, EA up-regulated extracellular ion contents to achieve persistent efficacy in gastric activity [50]. In line with our experience, EA/TEA can be initiated at 1 day after surgery.
Limitation
Certain limitations should be conducted in the present work. Firstly, high risk of bias was detected because of outcome evaluation blinding, participant/personnel blinding, and allocation concealment. Such high bias risk will reduce our data creditability. Secondly, most of our enrolled RCTs were conducted in mainland China, possibly leading to bias and result limitations. Therefore, further high-quality RCTs should be conducted for confirming EA/ TEAS’ therapeutic effect. Thirdly, there were obvious heterogeneity among RCTs after outcome analysis. In addition, the enrolled RCTs involved diverse control interventional measures and acupuncture manipulation procedures (needle position, timing, frequency, course of treatment). Besides, the standard EA/TEAS therapeutic protocol should be developed for minimizing the inter-study heterogeneity.
Conclusion
In conclusion, our meta-analysis results indicate the safety and efficacy of EA/TEAS in treating postoperative POI. EA/TEAS can reduce time-to-first flatus/defecation/bowel sound, reduce POI risk and decrease LOS. Moreover, difference in the efficacy in postoperative POI was not significant between EA and TEAS. In this regard, more large, long-time RCTs with different sample sizes are needed. In addition, the thorough indicators should be identified to verify our results. The present work lays certain foundation for patients with postoperative POI who seek the appropriate treatment and superior therapeutic outcome.
Data Availability
All the data supporting this meta-analysis are from previously reported studies and data sets, which have been cited. The processed data are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Funding Statement
This work was supported by the Traditional Chinese Medicine Bureau of Guangdong Province Science foundation(no.20193002).
Supplementary Materials
Table 1 provides the basic characteristics of included clinical EA or TEAS trials of POI treatment. Figure 2 provides the forest plot of time-to-first flatus in EA/TEA vs control. Figure 3 provides the forest plot of time-to-first bowel sound in EA/TEA vs control. Figure 4 provides the forest plot of time-to-first defecation in EA/TEA vs control. Figure 5 provides the forest plot of efficacy of postoperative Ileus in EA/TEA vs control. Figure 6 provides the forest plot of length of hospital stay in EA/TEA vs control.
References
- Neuman HB, Schrag D, Cabral C, Weiser MR, Paty PB, et al. (2007) Can differences in bowel function after surgery for rectal cancer be identified by the European organization for research and treatment of cancer quality of life instrument? Ann Surg Oncol 14(5): 1727-1734.
- Langenhoff BS, Krabbe PF, Wobbes T, Ruers TJ (2001) Quality of life as an outcome measure in surgical oncology. Br J Surg 88(5): 643-652.
- Bauer AJ, Boeckxstaens GE (2004) Mechanisms of postoperative ileus. Neurogastroenterol Motil 16 Suppl 2: 54-60.
- Wolff BG, Michelassi F, Gerkin TM, Techner L, Gabriel K, et al. (2004) Alvimopan, a novel, peripherally acting mu opioid antagonist: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg 240(4): 728-734.
- Wu Z, Boersema GS, Dereci A, Menon AG, Jeekel J, et al. (2015) Clinical endpoint, early detection, and differential diagnosis of postoperative ileus: A systematic review of the literature. Eur Surg Res 54(3-4): 127-138.
- Person B, Wexner SD (2006) The management of postoperative ileus. Curr Probl Surg 43(1): 6-65.
- Vather R, Trivedi S, Bissett I (2013) Defining postoperative ileus: Results of a systematic review and global survey. J Gastrointest Surg 17(5): 962-972.
- Kehlet H (2008) Postoperative ileus--an update on preventive techniques. Nat Clin Pract Gastroenterol Hepatol 5(10): 552-558.
- Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, et al. (2015) Underlying reasons associated with hospital readmission following surgery in the United States. Jama 313(5): 483-495.
- Venara A, Neunlist M, Slim K, Barbieux J, Colas PA, et al. (2016) Postoperative ileus: Pathophysiology, incidence, and prevention. J Visc Surg 153(6): 439-446.
- Fang JF, Fang JQ, Shao XM, Du JY, Liang Y, et al. (2017) Electroacupuncture treatment partly promotes the recovery time of postoperative ileus by activating the vagus nerve but not regulating local inflammation. Sci Rep 7: 39801.
- Kehlet H (2008) Fast-track colorectal surgery. Lancet 371(9615): 791-793.
- Asgeirsson T, El-Badawi KI, Mahmood A, Barletta J, Luchtefeld M, et al. (2010) Postoperative ileus: It costs more than you expect. J Am Coll Surg 210(2): 228-231.
- Van Bree SH, Nemethova A, Cailotto C, Gomez-Pinilla PJ, Matteoli G, et al. (2012) New therapeutic strategies for postoperative ileus. Nat Rev Gastroenterol Hepatol 9(11): 675-683.
- Cumpston M, Li T, Page MJ, Chandler J, Welch VA, et al. (2019) Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10: ED000142.
- Deng JJ (2010) The adjustment effect and mechanism of acupuncture on gastrointestinal motility disorders after abdominal surgery.
- Wang JW WX, Po JR (2010) Electroacupuncture for postoperative gastrointestinal function suppression in 57 cases. Shanxi TCM 26(58).
- Gan K (2010) Effect of electroacupuncture on recovery of gastrointestinal function in patients with PCIA analgesia with morphine after gynecological total hysterectomy.
- Yang JJ (2011) Effects of electroacupuncture at Xiahe point on gastrointestinal function in patients with colorectal cancer after surgery.
- Lu P, Zhou ZHL (2012) Electroacupuncture promotes the recovery of gastrointestinal function after abdominal surgery in 60 cases. Chinese Journal of Integrative Medicine Surgery 18(1).
- Zhen YH (2013) Clinical study on the effect of electroacupuncture at Zusanli on recovery of gastrointestinal motility after abdominal surgery.
- Wang TH (2014) Efficacy observation of electroacupuncture at points of Yangming meridian in the treatment of gastrointestinal dysfunction after abdominal surgery.
- Yang ZL, Guo YQ, Zhen RZ (2015) Effects of percutaneous electrical acupoint stimulation-assisted general anesthesia on gastrointestinal function after gynecological endoscopic surgery. Shaanxi TCM 36(12).
- Zhu W (2015) Effects of acupuncture on the recovery of gastrointestinal function after combined hysteroscopy and laparoscopy.
- Chen Hui QL, Huang YG, Xie DF (2015) Effects of early electroacupuncture at Xiahe point on the recovery of gastrointestinal function after bowel surgery. Integrative Medicine 12(15).
- Hu SS (2006) The effect of acupuncture and medicine intervention on the recovery of gastrointestinal function after abdominal hysterectomy.
- Wang PCL (2016) Clinical observation of electroacupuncture for gastrointestinal dysfunction after abdominal surgery. Shanghai Journal of Acupuncture and Moxibustion 35(12).
- Shen HX (2017) A preliminary study on electroacupuncture in promoting gastrointestinal function recovery after cesarean section.
- Yuan L, Tang W, Wang J, Fu GQ, Guo F (2017) Effects of percutaneous electrical acupoint stimulation on perioperative gastrointestinal function in laparoscopic bowel surgery. Journal of Clinical Anesthesiology 33(6).
- Gu SH (2019) Comparison of percutaneous electrical acupoint stimulation at different times in the perioperative period on analgesia and recovery of gastrointestinal function after laparoscopic radical gastrectomy.
- Mu L, Gao H, Zhao ML, Ren HF, Ma HS (2019) Effects of transcutaneous electrical acupoint stimulation on recovery of gastrointestinal function after cesarean section. Zhongguo Zhen Jiu 39(3): 259-262.
- Pu L, Wen YA (2019) randomized controlled study of the effect of electroacupuncture on the recovery of gastrointestinal function after abdominal surgery. Journal of Bengbu Medical College 44(5).
- Qu LZ, Shen HX, Yan Z, Ma SJ, Wang KQ (2020) Effects of single-point acupuncture and multi-point acupuncture on gastrointestinal function in patients after laparoscopic cholecystectomy under general anesthesia. Accupuncture Research 45(2).
- Feng DH (2020) Influence of percutaneous electrical acupoint stimulation preconditioning on postoperative gastrointestinal function rehabilitation in patients undergoing laparoscopic nodal surgery for cancer.
- Li KH (2020) Effects of transcutaneous electrical acupoint stimulation on the recovery of gastrointestinal function after gastric cancer surgery.
- Song K (2020) Effects of percutaneous electrical acupoint stimulation on gastrointestinal function in patients undergoing laparoscopic gastrectomy.
- Li F, Gan JH, Wang CZ, Cui XY, Shi JL, et al. (2020) Effects of percutaneous electrical acupoint stimulation on pain, gastrointestinal function and plasma inflammatory factors after laparoscopic radical resection of cervical cancer. Western Chinese Medicine 33(11).
- Zhang HL, Bai YB, Lu YQ (2020) Effects of transcutaneous electrical acupoint stimulation on postoperative bowel function in patients with colorectal tumors. Modern Oncology Med 28(04).
- Xu SG, Liu JL, Wu L (2020) Efficacy observation of electroacupuncture on the recovery of gastrointestinal function in patients after appendectomy. CJCM Clinical Research of Traditional Chinese Medicine 12(18).
- Zhou X, Cao SG, Tan XJ, Liu XD, Li ZQ, et al. (2021) Effects of Transcutaneous Electrical Acupoint Stimulation (TEAS) on postoperative recovery in patients with gastric cancer: A randomized controlled trial. Cancer Manag Res 13: 1449-1458.
- Xu JY (2021) The effect of transcutaneous electrical acupoint stimulation therapy device combined with comprehensive nursing care on the recovery of gastrointestinal function and prognosis after cesarean section. Medical Equipment 34(1).
- Li WJ, Gao C, An LX, Ji YW, Xue FS, et al. (2021) Perioperative transcutaneous electrical acupoint stimulation for improving postoperative gastrointestinal function: A randomized controlled trial. J Integr Med 19(3): 211-218.
- Liu X (2021) Progress of action mechanism and clinical study on acupuncture and moxibustion in promoting postoperative gastrointestinal function recovery of colon cancer. Beijing university of Chinese Medicine,100029 Beijing, China.
- Gao W, Li W, Yan Y, Yang R, Zhang Y, et al. (2021) Transcutaneous electrical acupoint stimulation applied in lower limbs decreases the incidence of paralytic ileus after colorectal surgery: A multicenter randomized controlled trial. Surgery 170(6): 1618-1626.
- Li JJ, Zhao WS, Shao XM, Yang AM, Zhang FF, et al. (2016) Effect of transcutaneous electrical acupoint stimulation on post-surgical gastrointestinal function, autonomic nerve activities and plasma brain-gut peptide levels in patients undergoing gastrointestinal surgery. Zhen Ci Yan Jiu 41(3): 240-246.
- Hou LW, Rong PJ, Li L, Wei W, Fang JL, et al. (2021) Effects of transcutaneous auricular vagus nerve stimulation on autonomic nervous function in rats with functional dyspepsia. Zhen Ci Yan Jiu 46(8): 663-670.
- Han X, Chen X, Wang X, Gong M, Lu M, et al. (2021) Electroacupuncture at ST36 improve the gastric motility by affecting neurotransmitters in the enteric nervous system in type 2 diabetic rats. Evid Based Complement Alternat Med, p. 6666323.
- Liang C, Wang K, Xu B, Yu Z (2016) Electroacupuncture at acupoint ST 37(Shangjuxu) improves function of the enteric nervous system in a novel mouse constipation model. BMC Complement Altern Med 16(1): 392.
- Zhu X, Liu Z, Qu H, Niu W, Gao L, et al. (2016) The effect and mechanism of electroacupuncture at LI11 and ST37 on constipation in a rat model. Acupunct Med 34(3): 194-200.
- Gao R, Gao S, Feng J, Cui H, Cui Y, et al. (2018) Effect of electroacupuncture on (99m) Tc-Sodium pertechnetate uptake and extracellular fluid free molecules in the stomach in acupoint ST36 and ST39. Sci Rep 8(1): 6739.
© 2022 Juan-Juan Zhang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






