- Submissions

Full Text
Gastroenterology Medicine & Research
Kombucha Liver Injury, A Strange Presentation: A Case Report
Labanca Sara1, Ziola Sebastiano1, Grillo Federica2, Marenco Simona1, Cacciato Valentina Lily Maria1, Pieri Giulia1, Lonati Davide3, Brambilla Elena3, Locatelli Carlo3, Picciotto Antonino1 and Borro Paolo4*
1Gastroenterology Unit, Department of Internal Medicine, IRCCS Osp. Policlinico San Martino, Genova, Italy
2Anatomic Pathology Unit, Department of Surgical Sciences and Integrated Diagnostics (DISC), University of Genoa, IRCCS Osp. Policlinico San Martino, Genova, Italy
3Pavia Poison Control Centre and National Toxicology Information Centre - Toxicology Unit, IRCCS Istituti Clinici Scientifici Maugeri, Pavia, Italy
4Hepatobiliopancreatic and Liver transplant Surgery Unit, IRCCS Policlinico San Martino, Genoa, Italy
*Corresponding author: Borro Paolo, Hepatobiliopancreatic and Liver transplant Surgery Unit, IRCCS Policlinico San Martino, Genoa, Italy
Submission:April 28, 2022;Published: May 10, 2022

ISSN 2637-7632Volume7 Issue1
Abstract
Background: Kombucha is a fermented tea commonly consumed for its supposed health benefits. However, some cases of toxicity are reported. To our knowledge there are no case reports of cholestasis.
Case Report: A 31-year-old man was admitted to our hospital for jaundice. Initially abdominal ultrasonography revealed cholelithiasis and choledocholithiasis. We performed Endoscopic Retrograde Cholangiopancreatography (ERCP) with gallstones removal and sphincterotomy, without any benefit on jaundice. After excluding all etiologies, a toxicological history revealed the consumption of Kombucha tea about three weeks before hospitalization. We started therapy with a high-dosage of N-acetylcysteine (300mg/kg, i.v.), Ursodeoxycholic acid-UDCA (15mg/kg) and fluids for 14 days. A progressive reduction of liver tests was shown.
Conclusion: Kombucha tea is widely used and it should be considered as a potential Drug-Induced Cholestasis (DIC)as well as some drugs or herbal supplementations.
Keywords: Kombucha; Liver injury; Cholestasis; Case report
Abbreviations: AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; ALP: Alkaline Phosphatase; gGT: Gamma-Glutamyl Transpeptidase, WBC: White Blood Cells; Hb: Hemoglobin; ERCP: Endoscopic Retrograde Cholangiopancreatography; MRCP: Magnetic Resonance Cholangiopancreatography
Introduction
Kombucha tea is an increasingly popular beverage made by fermenting tea (generally black tea or sometimes green or oolong tea) and sugar, with a Symbiotic Culture of Bacteria and Yeast (SCOBY). This process generally takes 7-10 days [1]. Although it is thought to be healthy and have therapeutic effects, there are some reports about its potential toxicity. To our knowledge there are no case reports of cholestasis.
Case Report
Chief complaints
A 31-year-old Caucasian man, obese (BMI 49), non-smoker, with no significant alcohol consumption was admitted to the Emergency Department for jaundice and high bilirubin levels total bilirubin 7,11 mg/dl, direct bilirubin 5,58 mg/dl.
History of present illness
Following the appearance of jaundice, an abdominal ultrasonography revealed cholelithiasis and dilatation of intrahepatic and common bile ducts due to choledocholithiasis. The patient underwent Endoscopic Retrograde Cholangiopancreatography (ERCP) with gallstones removal and sphincterotomy. In the following days, laboratory data showed a progressive increase of bilirubin levels with a concomitant rise of AST (Aspartate Aminotransferase), ALT (Alanine Aminotransferase), Alkaline Phosphatase (ALP) and Gamma- Glutamyl Transpeptidase (gGT) despite the endoscopic treatment. A Magnetic Resonance Cholangiopancreatography (MRCP) and a further ERCP were performed and both showed no significant alterations. The patient was transferred to the Hepatologic Unit. The toxicological and pharmacological history revealed the consumption of Kombucha tea about three weeks before hospitalization. The patient underwent a US-guided hepatic biopsy.
History of past illness
History of obesity
Physical examination
Jaundice and obesity (BMI 49)
Laboratory examinations
Laboratory tests showed: WBC (white blood cells) 8,26 x10^9/L, Hb (hemoglobin) 129g/L, Bilirubin 26.59mg/dL, direct bilirubin 23.8mg/dL, AST 78U/L, ALT 54U/L, ALP 102U/L, gGT 44U/L, albumin 33.9g/L, amilase 75U/L, lipase 205U/L, INR 1.23, creatinine 0.9. Autoimmune hepatitis markers (ANA, AMA, ASMA, P-ANCA, anti-LC1, anti-SLA/LP) and viral hepatitis markers were negative. Ceruloplasmin and Alpha 1 anti-trypsin were normal.
Imaging examinations
During hospitalization in the Hepatology Unit, the patient underwent an ultrasound examination which revealed diffuse steatosis and inhomogeneity. The dilatation of the biliary tract was no longer evident. Lithiasis of the gallbladder has been confirmed. Endoscopic Ultrasonography (EUS) was performed in order to exclude any obstructive etiologies.
Liver biopsy
Figure 1: a) Liver biopsy showing expansion of portal tracts by moderate inflammatory infiltrate and mild steatosis (haematoxylin and eosin, 4x magnification). b) Higher magnification of a portal tract with moderate inflammatory infiltrate composed of lymphocytes, plasmacells and scattered eosinophils – blue arrows (haematoxylin and eosin, 20x magnification). c) Hepatic parenchyma with diffuse canalicular cholestasis (black arrows). In particular, in the centrilobular area there is a mild steatosis, liver cell ballooning (black asterisk) and scattered parenchymal neutrophils (yellow arrow) in keeping with steatohepatitis (haematoxylin and eosin, 20x magnification). d) Trichrome stained section showing pericellular fibrosis in blue in the centrilobular area with liver cell ballooning (green arrow) and mildmoderate steatosis (Azan Mallory Trichrome, 20x magnification). The cholangitic lesions are not shown.
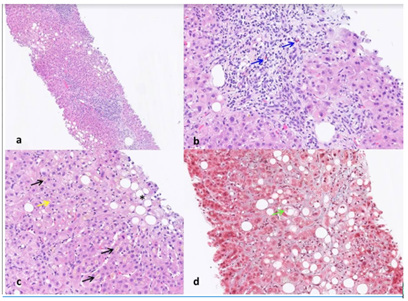
The patient underwent a US-guided hepatic biopsy and the histology (Figure 1) showed a complex pattern consisting of:
1. Alcoholic steatohepatitis (ASH)/ Non-Alcoholic steatohepatitis (NASH) (Stage 2 – Grade 2 sec. Brunt M. et al. 1999)
2. Acute cholangitic lesions (in resolution)
3. Marked canalicular cholestasis with scattered eosinophil granulocytes and ceroid laden Kupffer cells
Final Diagnosis
Kombucha induces severe cholestatic liver injury.
Treatment
In accordance with the Pavia Poison Control Centre (Pavia-PCC) we started therapy with a high-dosage of N-acetylcysteine (300mg/ kg, i.v), Ursodeoxycholic acid-UDCA (15mg/kg) and fluids for 14 days.
Outcome and follow-up
During hospitalization the patient continued to be asymptomatic, afebrile and with regular bowel function. No alterations in blood coagulation or signs of hepatic encephalopathy were identified. A progressive, although slow, reduction of bilirubin, AST, ALT levels with persistence of modest alterations of pancreatic enzymes was noticed. At discharge, the laboratory data showed: WBC 12,7 x 10^9, Hb 124g/L, AST 82U/L, ALT 69U/L, ALP 95U/L, gGT 59U/L, bilirubin 14.85mg/dL, direct bilirubin 12.2mg/dL, amylase 63U/L, lipase 173U/L. The patient also underwent a bariatric surgery visit and received a specific home diet. One month after hospitalization the patient came to visit and the laboratory data showed a complete normalization of the hepatic profile.
Discussion
Kombucha is a symbiotic aggregate of various yeasts and bacteria, usually including Saccharomyces sp. and, following fermentation, a cocktail of chemical components such as alcohol, ethyl acetate, acetic acid and lactate. Until now no case reports have established a direct correlation between Kombucha consumption and pathological conditions. Nevertheless, the Food and Drug Administration noted that kombucha might be produced under several conditions even in a domestic environment thus contamination with pathogenic organisms such as Aspergillus is possible [2]. In 1995, the Center for Disease Control and Prevention (CDC) published 2 case reports of suspected kombucha tea toxicity in 2 middle-aged women from Iowa [3]. Both patients presented sever lactic acidosis which led to cardiac arrest (one of them died). Both patients had consumed kombucha tea in the previous two months. A case of probable gastrointestinal effects (nausea and vomiting) by kombucha was described in 1997 [4,5]. In 1998 a case of cutaneous anthrax associated with kombucha consumption was described in Iran [6]: 20 patients who reported skin lesions typical for anthrax (evaluated by local specialists), had locally applied kombucha mushrooms on their skin to alleviate the pain; the composition was then analyzed, suggesting that kombucha tea seemed to be a good media for B. anthrax. In 2003 a case of anti-Jo1 myositis with pericardial tamponade after Kombucha exposure was reported [5-7]. Regarding liver toxicity, two cases of acute hepatitis after kombucha consumption were reported in 1996 and in 1997 [4,8]. In our case report we describe typical cholestatic damage with canalicular cholestasis documented by liver biopsy (Figure 1). We hypothesize that the targets of the hepatic damage are represented by the transport proteins involved in biliary homeostasis as described for some drugs responsible for DIC (drug-induced cholestasis) [7]. In conclusion, due to its growing popularity, kombucha consumption should be considered in cases of unexplained cholestatic liver damage. Treatment with N-acetylcysteine may be advisable in the case of liver toxicity by kombucha. Herbal hepatic toxicity may trigger damage in patients with a “healthy liver” but is also likely to be more deleterious in patients whose liver is affected by chronic diseases, such as steatohepatitis.
Acknowledgement
Author contributions
Labanca S highlighted the importance of the case and wrote the manuscript; Ziola S contributed writing the manuscript; Grillo performed the histological evaluation and discussed the case; Borro P contributed to the writing and revision of the manuscript; Marenco S and Pieri G contributed to the design and revision of the manuscript; Cacciato VLM is a native English speaker and contributed to the language editing and revision of the manuscript; Lonati D, Brambilla E and Locatelli C revised the manuscript; Picciotto A discussed the case and wrote the conclusion; all the authors have read and approved the final revised manuscript.
Informed consent statement
Informed written consent was obtained from the patient for publication of this report and any accompanying images. This study does not contain identifying information of the patient.
Conflict-of-interest statement
The authors declare that they have no competing or conflicts of interests.
References
- Kapp JM, Sumner W (2019) Kombucha: A systematic review of the empirical evidence of human health benefit. Ann Epidemiol 30: 66-70.
- Food and Drug Administration (1995) FDA cautions consumers on “Kombucha Mushroom Tea”. US Department of Health and Human Services, Public Health Service, Food and Drug Administration, Washington DC, USA.
- Center for Disease Control and Prevention (CDC) (1995) Unexplained severe illness possibly associated with consumption of Kombucha tea- Iowa, 1995. MMWR Morh Mortal Wkly Rep 44(48): 892-900.
- Srinivasan R, Smolinske S, Greenbaum D (1997) Probable gastrointestinal toxicity of Kombucha tea: Is this beverage healthy or harmful? Gen Intern Med 12(10): 643-645.
- Derk CT, Sandorfi N, Curtis MT (2004) A case of anti-Jo1 myositis with pleural effusions and pericardial tamponade developing after exposure to a fermented Kombucha beverage. Clin Rheumatol 23(4): 355-357.
- Sadjadi J (1998) Cutaneous anthrax associated with the Kombucha "mushroom" in Iran. JAMA 280(18): 1567-8
- Fernández-Murga ML, Petrov PD, Conde I, Castell JV, Goméz-Lechón MJ, et al. (2018) Advances in drug-induced cholestasis: Clinical perspectives, potential mechanisms and in vitro Food Chem Toxicol 120: 196-212.
- Perron AD, Patterson JA, Yanofsky NN (1995) Kombucha "mushroom" hepatotoxicity. Ann Emerg Med 26(5): 660-661.
© 2022 Borro Paolo. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






