- Submissions

Full Text
Gastroenterology Medicine & Research
Progressive Thrombocytopenia and Clinical Deterioration with Initial Diagnosis of Esophageal Cancer-Secondary Hemophagocytic Lymphohistiocytosis as a Complicating Cause
Mani Nassir1*, Adrian Schreiber2, Georg Hilfenhaus1, Sebastian Stintzing1 and Uwe Pelzer1
1Department of Hematology, Oncology and Tumor Immunology (CCM), Charity-University medicine Berlin, Berlin, Germany
2Department of Nephrology and Medical Intensive Care, Charity-University medicine Berlin, Berlin, Germany
*Corresponding author: Mani Nassir, Department of Hematology, Oncology and Tumor Immunology (CCM), Charity- University medicine Berlin, Berlin, Germany
Submission:March 18, 2022;Published: March 29, 2022

ISSN 2637-7632Volume6 Issue5
Case Report
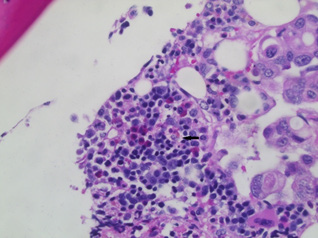
A 52-year-old male patient presented in the emergency department with fatigue and persistent back and flank pain. Computed tomography revealed lymphadenopathy and disseminated osseous lesions. Staging was completed and a low differentiated signet ring cell carcinoma of the distal esophagus (AEG I), UICC stage IV [1], was diagnosed. Immunohistochemistry showed expression of human epidermal growth factor receptor 2 (HER2)/neu and E-cadherin without expression of programmed death-ligand [1]. No microsatellite instability was detected. Palliative polychemotherapy with 5-fluorouracil, folinic acid, oxaliplatin and docetaxel (FLOT) was initiated. Due to progressive pancytopenia with transfusion-dependent thrombocytopenia, abnormal renal function tests, fever and rapid clinical deterioration, we suspected malignancy-associated Thrombotic Microangiopathy (TMA), and therefore promptly initiated daily plasmapheresis. However, no objective response upon plasmapheresis was observed, while extended laboratory tests revealed elevated levels of lactate dehydrogenase (1477U/l, range: 135-250U/l), ferritin (4410μg/l, range 30- 400μg/l), triglycerides (366mg/dl, range >200mg/dl) and soluble interleukin 1 receptor (1807IU/ml, range: <710IU/ml). Abdominal ultrasonography revealed splenomegaly. Notably, bone marrow biopsy revealed extensive carcinomatosis and activation of stromal macrophages (Figure 1). According to those findings and a calculated H-score (reference: http://saintantoine.aphp.fr/score/), malignancy associated Secondary Hemophagocytic Lymphohistiocytosis (sHLH) was diagnosed. Thus, we initiated treatment with interleukin (IL)-1 inhibition (Anakinra), prednisolone and continuation of polychemotherapy, resulting in a significant clinical improvement and decline of ferritin (1990.4μg/l) and lactate dehydrogenase (860U/l) levels. Subsequently, the patient successfully completed six cycles of polychemotherapy but eventually died due to progressive esophageal carcinoma. What do we learn from this unusual case? First, distinguishing sHLH from TMA in the context of rapid onset thrombocytopenia along with clinical and laboratory deterioration is extremely challenging. However, extensively elevated ferritin levels are indicative for HLH and may guide diagnostic considerations early on [2]. Second, Bone Marrow Carcinomatosis (BMC) due to gastric or esophageal carcinoma is a highly rare event [3,4], and concomitant sHLH is a yet underreported, but potentially life-threatening phenomenon in this context. HLH is a hyperinflammatory syndrome leading to an uncontrolled cytokine storm with an all-cause mortality of approximately 40% in adults [5]. Although exact path mechanisms remain unclear, dysregulation of immune homeostasis due to chemotherapy and underlying malignancy might lower the threshold for triggering sHLH [6]. However, in this patient we considered BMC as driver of sHLH and therefore decided to continue chemotherapy. Third, therapeutic decision-making remains challenging due to a lack of validated treatment protocols in adults with sHLH. In a recent report from China, two gastric cancer patients with sHLH were treated with glucocorticoids plus etoposide and etoposide/doxorubicin, respectively, but after clinical recovery sHLH relapsed [7]. As IL-1 is crucial in the pathogenesis of HLH, we opted for subcutaneous application of anakinra-no adverse events occured and our patient quickly recovered from sHLH.
Figure 1: Carcinomatosis of the bone marrow with remaining haematopoiesis and activation of stromal macrophages. Shown is a representative hematoxylin and eosin staining of the bone marrow biopsy (40x magnification). An activated stromal macrophage is indicated by the arrow.
Conclusion
In conclusion, considering sHLH in patients with solid tumors and identifying BMC as a potential trigger, remains challenging in the clinical routine. Once the diagnosis is established, anakinra is an effective and well tolerated first line treatment option with a favorable benefit-risk profile.
Conflict of Interest
All authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was not obtained from the patient referenced in the case report (deceased). Multiple attempts were made to reach his next of kin to obtain consent, but we were unable to make contact with his family.
References
- Zhang F, Luo W, Shi Y, Fan Z, Ji G (2012) Should we standardize the 1,700-year-Old fecal microbiota transplantation? Am J Gastroenterol 107(11): 1755.
- Vishwakarma R, Goswami PK (2013) A review through charka Uttara-tantra. Ayu 34(1): 17-20.
- https://es.wikipedi.org/wiki/Historia de la medicine traditional china
- Abreu AT (2018) Fecal microbiota transplant. Pre-congress course on gastroenterology. National gastroenterology week 2018. Practical answers to frequent and complex problems in gastroenterology, p. 367.
- McComick J (2016) German soldiers forced to eat poop to cure dysentery outbreak. War History Online.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ (1958) Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44(5): 854–859.
- Farooq PD, Urrunaga NH, Tang DM, von Rosenvinge EC (2015) Pseudomembranous colitis. Dis Mon 61(5): 181-206.
- Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, et al. (2016) Epidemiological and economic burden of Clostridium difficile in the United States: Estimates from a modeling approach. BMC Infect Dis 16: 303.
- McCune VL, Quraishi MN, Manzoor S, Moran CE, Banavathi K, et al. (2020) Results from the first English stool bank using faecal microbiota transplant as a medicinal product for the treatment of Clostridioides difficile EClinicalMedicine 20: 100301.
- Van Lingen E, Terveer EM, van der Meulen-de-Jong AE, Vendrik KE, Verspaget HW, et al. (2019) Advances un stool banking. Microb Health Dis 2: e182.
- Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, et al. (2019) International consensus conference on stool banking for faecal Microbiota transplantation in clinical practice. Gut 68(12): 2111-2121.
- Jørgensen SM, Hvas CL, Dahlerup JF, Nikkelsen S, Ehlers L, et al. (2019) Banking feces: A new frontier for public blood banks? Transfusion 59(9): 2776-2782.
- Kim KO, Gluck M (2019) Fecal microbiota transplantation: An update on clinical practice. Clin Endosc 52(2): 137-143.
- Ramai D, Zakhia K, Ofosu A, Ofori E, Reddy M (2019) Fecal microbiota transplantation: Donor relation, fresh or frozen, delivery methods, cost-effectiveness. Ann Gastroenterol 32(1): 30-38.
- Woodwort MH, Neish EM, Miller NS, Dhere T, Burd EM, et al. (2017) Laboratory testing of donors and stool samples for fecal microbiota transplantation for recurrent Clostridium difficile I J Clin Microbiol 55(4): 1002-1010.
- Moossavi S, Bishehsari F, Ansari R, Vahedi H, Nasseri-Moghaddam S, et al. (2015) Minimum requirements for reporting fecal microbiota transplant trial. Middle East J Dig Dis 7(3): 177-180.
- Duvallet C, Zellmer C, Panchal P, Budree S, Osman M, et al. (2019) Framework for rational donor selection in fecal microbiota transplant clinical trials. PLoS One 14(10): e0222881.
- Haifer C, Kelly CR, Paramsothy S, Andresen D, Papanicolas LE, et al. (2020) Australian consensus statements for the regulation, Production And use of faecal microbiota transplantation in clinical practice. Gut 69(5): 801-810.
- Van Beurden H, Groot PF, van Nood E, Niewdorp M, Keller JJ, et al. (2017) Complications, effectiveness, and long term follow-up of fecal microbiota transfer by nasoduodenal tube for treatment of recurrent Clostridium difficile United European Gastroenterol J 5(6): 868-879.
- Krumbeck JA, Rasmussen HE, Hutkins RW, Clarke J, Shawron K, et al. (2018) Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 6(1): 121.
- Wang S, Xu M, Wang W, Cao X, Piao M, et al. (2016) Systematic review: Adverse events of fecal microbiota transplantation. PloS One 11(8): e0161174.
- Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, et al. (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nature Reviews Gastroenterology & Hepatology 17: 687-701.
- Choi HH, Cho YS (2016) Fecal microbiota transplantation: Current applications, effectiveness, and future perspectives. Clin Endosc 49(3): 257-265.
- König J, Siebenhaar A, Högenauer C, Arkkila P, Nieuwdorp M, et al. (2017) Consensus report: Faecal microbiota transfer- clinical applications and Procedures. Aliment Pharmacol Ther 45(2): 222-239.
- Zhou Y, Xu H, Huang H, Li Y, Chen H, et al. (2019) Are there potential applications of fecal microbiota transplantation beyond intestinal disorders?. Biomed Res Int: 3469754.
- Merrick B, Allen L, Zain NM, Forbes B, Shawcross DL, et al. (2020) Regulation, risk and safety of faecal microbiota transplant. Infect Prev Pract 2(3): 100069.
- Dang X, Xu M, Liu D, Zhou D, Yang W (2020) Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: A systematic review and meta-analysis. PLoS One 15(3): e0228846.
- Kelly CR, Ihunnah C, Fisher M, Khoruts A, Surawicz C, et al. (2014) Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 109(7): 1065-1071.
- Shogbesan O, Poudel DR, Victor S, Jehangir A, Fadahunsi O, et al. (2018) Systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can J Gastroentrrol Hepatol: 1394379.
- Kragsnaes MS, Kjeldsen J, Horn HC, Munk HL, Pedersen FM, et al. (2018) Efficacy and safety of faecal microbiota transplantation in patients with psoriatic arthritis: Protocol for a 6-month, double-blind, randomised, placebo-controlled trial. BMJ Open 8(4): e019231.
- Quaraishi MN, Widlak M, Bhala N, Moore D, Price M, et al. (2017) Systematic review with meta‐analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile Aliment Pharmacol Ther 46(5): 479-493.
- Cheng YW, Fisher M (2017) Fecal microbiota transplantation in the elderly: A need for early consideration in select cases of Clostridium difficile Practical Gastroenterology, pp. 16-22.
- Hourigan S, Oliva-Hemker M (2016) Fecal microbiota transplantation in children. A brief review. Pediatr Res 80(1): 2-6.
- Al-Jashaami LS, DuPont HL (2016) Management of Clostridium difficile Gastroenterol Hepatol (N Y) 12(10): 609-616.
- Davidovics ZH, Michail S, Nicholson MR, Kociolek LK, Pai N, et al. (2019) Fecal microbiota transplantation for recurrent Clostridium difficile infection and other conditions in children: A joint position paper from the north American society for pediatric. gastroenterology, hepatology, and nutrition and the European society for pediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr 68(1): 130-143.
- Zhong S, Zeng J, Deng Z, Jiang L, Zhang B, et al. (2019) Fecal microbiota transplantation for refractory diarrhea in immunocompromised diseases: A Pediatric case report. Ital J Pediatr 45(1): 116.
- Adisa R, Orherhe OM, Fakeye TO (2018) Evaluation of antibiotic prescriptions and use in under-five children in Ibadan, South Western Nigeria. Afr Health Sci 18(4): 1189-1201.
- Gurrama B, Sueb PK (2019) Fecal microbiota transplantation in children. Curr Opin Pediatr 31(5): 623-629.
- Giles EM, D´Adamo GL, Forster SC (2019) The future of faecal transplants. Nature Reviews Microbiology 17(12): 719.
- Leshem A, Horesh N, Elinav E (2019) Fecal microbial transplantation and its potential application in cardiometabolic syndrome. Front Immunol 10: 1341.
- Franklin CL, Ericsson AC (2017) Microbiota and reproducibility of rodent models. Lab Anim (NY) 46(4): 114-122.
- Rosebaum JT (2019) Just another crappy commentary: The future of fecal microbiota transplantation. Expert Rev Clin Immunol 15(10): 987-989.
- Bunnik EM, Aarts N, Chen LA (2017) Transplantation to ensure informed consent. The American Journal of Bioethic 17(5): 61-63.
- Bibbò S, Ianiro G, Gasbarrini A, Cammarota G (2017) Fecal microbiota transplantation: Past, present and future perspectives. Minerva Gastroenterol Dietol 63(4): 420-430.
© 2022 Mani Nassir. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






