- Submissions

Full Text
Gastroenterology Medicine & Research
New JAG1 Mutation and Rare Giant Hepatic Regenerate Nodule in Alagille Syndrome: A Case Report
Xiaohe Li1, Rui Huang1, Feng Liu1, Xiaoxiao Wang1, Lai Wei1,2 and Huiying Rao1*
1Peking University People’s Hospital, Peking University Hepatology Institute, Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Diseases, Beijing International Cooperation Base for Science and Technology on NAFLD Diagnosis, Beijing, China
2Beijing Tsinghua Changgung Hospital, Tsinghua University, Beijing, China
*Corresponding author: Huiying Rao, Peking University People’s Hospital, Peking University Hepatology Institute, Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Diseases, Beijing International Cooperation Base for Science and Technology on NAFLD Diagnosis, China
Submission:January 10, 2022;Published: January 20, 2022

ISSN 2637-7632Volume6 Issue4
Introduction
Alagille syndrome is a multisystem disease with intrahepatic bile duct paucity, chronic cholestasis, cardiac murmur, butterfly-like vertebra, posterior embryotoxon, and characteristic facial features. The estimated incidence is 1/30000 population based on the molecular diagnosis. A 15-year-old Chinese man was diagnosed with Alagille syndrome due to JAG1 deletion mutation, chronic cholestasis, facial characteristic and bile duct paucity. The deletion mutation c2519delA in JAG1 gene was confirmed by whole exome sequencing and was unreported before. Abdominal enhanced magnetic resonance imaging (MRI) showed a giant hepatic regenerative nodule adjacent to the right portal vein, with its branch crossing through. Pathology revealed ductular reaction and hepatocyte ballooning in hepatic nodule, was different from bile duct paucity in liver parenchymal. Our case broadens new pathogenic mutation in JAG1 and reports the existence of a giant regenerative nodule in Alagille syndrome patient, which were not mentioned in previous studies in the Chinese population.
Case Report
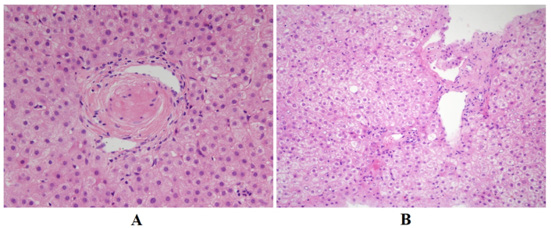
A 15-year-old male Chinese patient admitted because of repeating evaluated GGT for 1 year. He had significant jaundice in one year’s old and was treated by blood transfusion and had a history of recurrent otitis media for nearly 10 years. His parents and younger brother didn’t have similar symptom. On physical examination, he had a similar triangular face, broad forehead, pointed chin and splenomegaly. With further investigation, spina bifida occulta was diagnosed by radiography. Abdominal enhanced Magnetic Resonance Imaging (MRI) showed a solitary giant hepatic nodule (diameter: 5.7×8.3cm) was located adjacent to the right portal venous with vessels coursing through (Figure 1A-1F). Laboratory workup revealed significant elevated GGT (1595U/L) and slightly evaluated ALT and AST. Bile duct paucity (Figure 2A) was confirmed in the liver parenchyma according to pathology, and ductular reaction and hepatocyte ballooning were seen in the portion of hepatic nodule, indicated which was a regenerated nodule (Figure 2B). Echocardiogram, ophthalmic evaluation, renal ultrasonography, and renal function were normal. Whole exome sequencing genetic analysis confirmed a heterozygous gene mutation c.2519delA in JAG1, the deletion mutation induced amino change as p. N840Mfs*30, was recognized as “disease causing” by Mutation Taster (http://www.mutationtaster.org/). We also verified gene analysis for the patient’s father and younger brother, in whom we did not found similar mutation. His mother’s gene information was not obtained because his parents were divorced. Given the prominent feature of chronic cholestasis, our patient was treated with UDCA 13mg/kg, which consequently improved his liver function in the later follow-up.
Figure 1: Abdominal enhanced MRI of liver and giant hepatic nodule.
(A, B): The mass was isointense to the adjacent liver on T1 and fat-saturated T2-weighted.
(C, D): The mass enhanced similarly to the surrounding hepatic parenchyma in multi-phase enhancement.
(E, F): Origins of the celiac and superior mesenteric artery were severely narrowed, while the supplying organs did not have signs of ischemia.
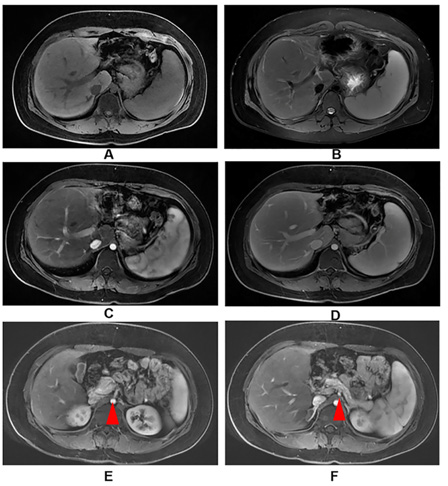
Figure 2: Pathology of liver parenchyma and hepatic nodule.
(A): Hematoxylin-eosin staining (original magnification, ×400) showing a portal tract with portal vein and hepatic artery but no bile duct in the surrounding liver parenchyma.
(B): Hematoxylin-eosin staining (original magnification, ×200) showing ductal reaction and hepatocyte ballooning within the regenerative nodule.
Discussion
In classical criteria of Alagille syndrome, confirmation of diagnosis should include bile duct paucity and three of five classical features mentioned before. Kamath et al. [1] proposed revised criteria adding with gene test. Our case had JAG1 mutation, bile duct paucity in liver biopsy, characteristic facies and chronic cholestasis and met the diagnostic criteria. In the present case, c.2519delA was first reported in our case, while the same variant was not found in familial verification of his father and younger brother, predicting that it was a de novo mutation. in the coding sequence. The newly JAG1 deletion mutation induced secondary frameshift mutation, then changed AA sequence and protein features, was recognized as disease-causing. According to the ACMG guideline, the loss function JAG1 is a known mechanism of Alagille syndrome was graded as pathogenic and weighted as very strong (PSV1) [2].
What’s more, to our surprise, the giant nodule in the liver adjacent to the right portal venous was recognized as hepatic regenerative nodule according to imaging and pathology. Few previous literatures reported, this may be considered a benign characteristic with typical location and vessels coursing through [3,4]. Comparative analysis of pathology features between the nodule and remainder of the liver parenchyma showed less damaged liver and visible interlobular bile ducts within the mass with unknown mechanism. A possible reason is the differential blood flow related to microvascular anomalies or variable geographical phenotypic expression of the JAG1 mutation in the liver. Notably, JAG1 was implicated in multiple forms of cancers, including hepatocellular carcinoma, and HCC was also reported to occur in Alagille syndrome patients [5]. We should still closely monitor the hepatic nodule in our patient.
Financial Support
National Natural Science Foundation of China (81870406).
References
- Kamath BM, Spinner NB, Piccoli DA (2007) Liver disease in children: Alagille syndrome. Cambridge: Cambridge University press, UK, pp. 326-345.
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB (2003) Consequences of JAG1 mutations. J Med Genet 40(12): 891-895.
- Roberts P, Trout AT, Dillman JR (2018) Nodular macro regenerative tissue as a pattern of regeneration in cholangiopathic disorders. Pediatr Radiol 48(7): 932-940.
- Alhammad A, Kamath BM, Chami R, Ng VL, Chavhan GB (2016) Solitary hepatic nodule adjacent to the right portal vein: A common finding of Alagille syndrome? J Pediatr Gastroenterol Nutr 62(2): 226-232.
- Tsai S, Gurakar A, Anders R, Lam-Himlin D, Boitnott J, et al. (2010) Management of large hepatocellular carcinoma in adult patients with Alagille syndrome: A case report and review of literature. Dig Dis Sci 55(11): 3052-3058.
© 2022 Huiying Rao. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






