- Submissions

Full Text
Gastroenterology Medicine & Research
Alcoholic Chronic Pancreatitis or Intraductal Papillary Mucinous Neoplasm: What to do?
Salvatore Fabio Rizzi, Silvia Mazzuoli and Francesco William Guglielmi*
Department of Gastroenterology and Artificial Nutrition, Italy
*Corresponding author: Francesco William Guglielmi, Department of Gastroenterology and Artificial Nutrition, Italy
Submission:June 12, 2020;Published: December 21, 2021

ISSN 2637-7632Volume6 Issue4
Abstract
Intraductal Papillary Mucinous Neoplasm (IPMN) is an intraductal mucin-producing neoplasm, with an increasing incidence. IPMNs may have clear malignant potential and exhibit a broad histological spectrum ranging from adenoma to invasive carcinoma. In contrast to the ductal adenocarcinoma, IPMNs have in general a better clinical prognosis. The clinical presentation of IPMN and Chronic Pancreatitis (CP) are often indistinguishable. Misdiagnosis of IPMN in patients with CP can lead to serious delays in the appropriate management. In patients with history of alcoholic CP, the possible presence of IPMN could not to be excluded. Due the high frequency of malignancy in IPMN, surgical approach should be considered. Assessment for potential IPMN is mandatory in patients with CP. All patients with CP must have a clinical assessment at least every 6 months, with abdominal US at least every year. In symptomatic patients with IPMN and severe abdominal pain, early pancreaticoduodenectomy must be strongly considered.
Introduction
Core tip
Clinical presentation of Intraductal Papillary Mucinous Neoplasm (IPMN) and Chronic Pancreatitis (CP) are often indistinguishable. Abdominal pain, jaundice, weight loss, nausea and vomiting are often seen in both conditions. Furthermore, CP can lead to formation of pseudocysts, that can have US and radiological features similar to IPMN, with considerable difficulty in differential diagnosis. Current guidelines recommend a waiting strategy for most types of pancreatic cysts. To avoid delays in the appropriate management of a potential malignant disease, we suggest an early surgical approach in symptomatic patients with pancreatic cyst and severe abdominal pain.
Intraductal Papillary Mucinous Neoplasm (IPMN) is an entity, first recognized in 1982, that is now in increasing attention. According to the WHO classification, IPMN is defined as an intraductal mucin-producing neoplasm of the main pancreatic duct and/or side branches, with variable degrees of papillary formation, mucin production, and cystic dilation [1]. IPMNs represent 20-50% of all pancreatic cystic neoplasms [2], but only about 1% of all pancreatic cancers [3]. Since IPMNs can be small and asymptomatic, the true incidence of this type of neoplasm is unknown, but the frequency with which IPMNs are being diagnosed worldwide is increasing [2,4]. In fact, Klibansky et al. performed a retrospective cohort study from 1985 to 2005 demonstrating an increased incidence of IPMNs due to an increase in diagnostic scanning rather than greater number of patients with clinically relevant disease [4]. IPMNs may have clear malignant potential and exhibit a broad histological spectrum ranging from adenoma to invasive carcinoma [5,6]. The disease progression is not clearly understood but has been hypothesized to follow the more well defined pancreatic ductal adenocarcinoma; IPMN adenoma to borderline IPMN with dysplasia to IPMN with carcinoma in situ and finally to invasive IPMN [7-9]. This “hyperplasia-dysplasia-carcinoma sequence” in the evolution of IPMNs is very similar to the “adenoma-carcinoma sequence” of colorectal tumours [10]. The time of progression from adenoma to invasive carcinoma appears to be slow but up to 30% of noninvasive IPMN may eventually become invasive and metastasize [7,11]. In contrast to the ductal adenocarcinoma, IPMNs have in general a better clinical prognosis with a 5-year survival of 77% for noninvasive IPMN and 43% for invasive IPMN with no difference between the different forms of noninvasive IPMN [7].
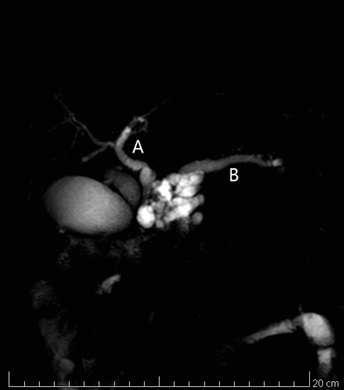
The clinical presentation of IPMN and Chronic Pancreatitis (CP) are often indistinguishable. Abdominal pain, jaundice, weight loss, nausea and vomiting are often seen in both conditions [12,13]. Furthermore, CP can lead to formation of pseudocysts, that can have US and radiological features similar to IPMN, with considerable difficulty in differential diagnosis. The misdiagnosis of IPMN in patients with CP can lead to serious delays in the appropriate management. The following case discussion highlights the interrelationship between IPMN and CP and illustrates the challenges in establishing an early diagnosis of IPMN. A 68-years-old white man initially presented in 2011 with CP. The principal symptoms were epigastric pain, nausea and weight loss. He was a hard smoker, high alcohol consumer, with history of lambda light chain monoclonal gammopathy, artificial heart valve implantation for previous stroke, hepatic steatosis, biliary sludge, previous streptococcal meningoencephalitis. No history of diabetes. In 2015, during US follow-up, evidence of a cystic multilocular lesion of the pancreas. TC (Figure 1) and Magnetic Resonance cholangiopancreatography imaging (Figure 2) showed a cystic multilocular lesion of about 4 centimeters diameter, in communication with the main pancreatic duct, that appeared dilated in the entire course, with dilatation of extrahepatic biliary ducts, overextended cholecystic, biliary sludge and enlarged peripancreatic lymph nodes. Endoscopic ultrasound confirmed the presence of a multilocular lesion, of about 4 cm diameter, in communication with the main pancreatic duct, compatible with intraductal papillary mucinous tumor. From 2011 to 2015, tumor markers were persistently normal, amylase and lipase were normal or slightly increased. Due to the persistence of severe epigastric pain, reductions of nutrient intake and worsening weight loss despite the use of oral nutritional supplements, the patient was referred to surgery unit and underwent a pancreaticoduodenectomy. Histology revealed a multilocular IPMN, located near major duodenal papilla (papilla of Vater), with high grade dysplasia (in situ carcinoma) and finite microinvasive characteristics. Immunohistochemistry pattern was: CK8/18 (+++), CK19 (++), CK7 (++-), Ki67 (5%). Histology of remaining pancreatic parenchyma showed a subacute pancreatitis or CP with areas of lithic necrosis. After a follow-up of 40 months, the patient is actually free of symptoms, with no signs of recurrence and no diabetes. The incidence of IPMN is dramatically increasing worldwide. IPMNs are potentially malignant neoplasms, graded to low-grade dysplasia (adenoma), moderate dysplasia (borderline), high-grade dysplasia (carcinoma in situ) and invasive carcinoma [2]. IPMNs can be anatomically classified as Main Duct (MD)-type, Branch Duct (BD)-type or mixed-type [14,15]. Most clinical series reported MD-type is characterized by much higher malignancy rates and worse prognosis than BD-types [2,16]. The mean frequency of malignancy in MD-IPMN is 61,6%, the mean frequency of invasive IPMN is 43,1% and the 5-year survival rates is 31-54% [17-19].
Figure 1: Abdominal computed tomography showed non-homogeneous cystic mass in the head of pancreas.
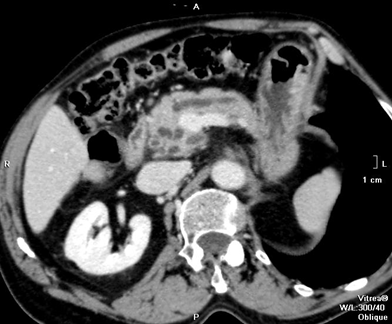
Figure 2: A. Magnetic Resonance cholangiopancreatography imaging showing the multilocular cyst, the common bile duct B. The main pancreatic duct.
Recognizing the different epidemiological factors to distinguish CP and IPMN is notably important. Age (63±11 years), female gender, low to moderate alcohol consumption and low to moderate smoking history appear to be the most significant factors for IPMN. Patients with CP are commonly males, 20 years younger, with high alcohol consumption and high smoking history [20]. Our patient had a history of very high alcohol abuse, leading to characterize the pathology as alcoholic CP, a history of severe abdominal pain unresponsive to specific therapy and the EUS evidence of a cystic lesion of about 4 centimeters diameter. Although in our patient risk factors can lean for an alcoholic CP, the possible presence of IPMN could not to be excluded. In fact, Petrou et al. discussed two cases of CP in patients without risk factors, who later were diagnosed with IPMNs [21]. For patients with cystic lesion >3cm, without high-risk stigmata of malignancy (obstructive jaundice, enhancing solid component within cyst or main pancreatic duct ≥10mm in size), guidelines suggest close surveillance (alternating MRI with EUS every 3-6 months) and to consider surgery in young, fit patients. In fact, due the high frequency of malignancy in IPMN, surgical approach should be considered. Guidelines do not suggest different management of IPMN in patients with underlying CP or clinical history of alcohol abuse. However, Traverso and al. demonstrated a greater risk of malignancy in IPMN when there is a clinical history of alcohol abuse [22]. For all the above, rather than to submit our patient to close surveillance, we decided that surgical resection was the optimal strategy for him. Histology showed IPMN with high grade dysplasia, confirming the adequacy of a decisive surgical intervention. Our experience suggests that assessment for potential IPMN is mandatory in patients with alcoholic CP. In case of cystic lesion occurrence suspected for IPMN, in young patients with alcoholic CP, early pancreaticoduodenectomy must be strongly considered.
References
- Adsay NV, Fukushima N, Furukawa T, Hruban RH, Klimstra DS, et al. (2010) Intraductal neoplasm of the pancreas. In: Bosman FT, Carneiro F, (eds.), WHO classification of tumors of digestive system. Lyon: WHO Press, pp. 304-313.
- Grützmann R, Niedergethmann M, Pilarsky C (2010) Intraductal papillary mucinous tumors of the pancreas: Biology, diagnosis and treatment. Oncologist 15(12): 1294-1309.
- Freeman HJ (2008) Intraductal papillary mucinous neoplasms and other pancreatic cystic lesions. World J Gastroenterol 14(19): 2977-2979.
- Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB (2012) The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol 10(5): 555-558.
- Scoazec J-Y, Vullierme M-P, Barthet M, Gonzalez J-M, Sauvanet A (2013) Cystic and ductal tumors of the pancreas: Diagnosis and management. J Visc Surg 150(2): 69-84.
- Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, et al. (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12(3): 183-197.
- Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, et al. (2004) Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg 239(6): 788-97.
- Cho KR, Vogelstein B (1992) Genetic alterations in the adenoma-carcinoma sequence. Cancer 70(6): 1727-1731.
- Bassi C, Sarr MG, Lillemoe KD, Reber HA (2008) Natural history of Intraductal Papillary Mucinous Neoplasms (IPMN): current evidence and implications for management. J Gastrointest Surg 12(4): 645-650.
- Wada K, Takada T, Yasuda H, Amano H, Yoshida M, et al. (2004) Does “clonal progression” relate to the development of intraductal papillary mucinous tumors of the pancreas?. J Gastrointest Surg 8(3): 289-296.
- Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, et al. (2002) Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology 123(5): 1500-1507.
- Klöppel G, Adsay NV (2009) Chronic pancreatitis and the differential diagnosis versus pancreatic cancer. Arch Pathol Lab Med 133(3): 382-387.
- Abu-Hilal M, Salvia R, Casaril A, Pearce NW, Bassi C, et al. (2006) Obstructive chronic pancreatitis and/or intraductal papillary mucinous neoplasms (IPMNs): A 21-year long case report. JOP: J Pancreas 7(2): 218-221.
- Kobari M, Egawa S, Shibuya K (1999) Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg 134(10): 1131-1136.
- Terris B, Ponsot P, Paye F (2000) Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol 24(10): 1372-1377.
- Nagai K, Doi R, Kida A, Kami K, Kawaguchi Y, et al. (2008) Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J Surg 32(2): 271-278.
- Suzuki Y, Atomi Y, Sugiyama M, Isaji S, Inui K, et al. (2004) Cystic neoplasm of the pancreas: A Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas 28(3): 241-246.
- Lee SY, Lee KT, Lee JK, Jeon YH, Choi D, et al. (2005) Long-term follow up results of intraductal papillary mucinous tumors of pancreas. J Gastroenterol Hepatol 20(9): 1379-1384.
- Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, et al. (2008) Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg 143(7): 639-646.
- Talamini G, Zamboni G, Salvia R, Capelli P, Sartori N, Casetti L, et al. (2006) Intraductal Papillary Mucinous Neoplasms and Chronic Pancreatitis. Pancreatology 6(6): 626-634.
- Petrou A, Papalambros A, Brennan N, Prassas E, Margariti T, et al. (2011) Intraductal Papillary Mucinous Neoplasm (IPMN) and chronic pancreatitis: Overlapping pathological entities? Two case reports. JOP 12(1): 50-54.
- Traverso LW, Peralta EA, Ryan JA Jr, Kozarek RA (1998) Intraductal neoplasms of the Pancreas. Am J Surg 175(5): 426-432.
© 2021 Francesco William Guglielmi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






