- Submissions

Full Text
Gastroenterology Medicine & Research
The Old, The New and The Future in Fecal Microbiota Transplantation
Álvaro Zamudio Tiburcio1* and Silverio Alonso López2
1Gastroenterologist, Intestinal Microbiota Transplantation, Mexico
2Urologist, Chairman Medical Specialties Naples in Mexico City, Mexico
*Corresponding author:Álvaro Zamudio Tiburcio, Gastroenterologist, Intestinal Microbiota Transplantation, Mexico
Submission: July 27, 2021;Published: August 16, 2021

ISSN 2637-7632Volume6 Issue1
Abstract
Determining the time that the Intestinal Microbiota can be conserved, without losing its efficiency, as well as evaluating the different aspects of Fecal Microbiota Transplantation, is what is intended in this work. First, the history of 2000 years to date is analyzed, regarding Microbiota Transplants, or is it 3000? And the following aspects are defined: Where did the Microbiota Transplant begin; where it was institutionalized, how many transplants have been performed, its costs. The important aspects of Recurrent Clostridiodes difficile Infection. The Intestinal Microbiota, its names; something about dysbiosis. The Methodology to carry out the IMT, excelling the donors. A list of the Conditions that improve with the Transplant is proposed. The stability time; The aim is to determine if it is necessary to use antibiotics before transplantation. The need for metagenomic and metatranscriptomic analyzes is considered. Finally, complications are evaluated and what could happen both in the immediate and distant future is proposed.
Abbreviations: FMT: Fecal Microbiota Transplantation; IM: Intestinal Microbiota; IMT: Intestinal Microbiota Transplantation
Introduction
The Fecal Microbiota Transplantation (FMT) was practiced for the first time by Ge Hong, a Chinese Alchemist, more than 1,700 years ago [1], although some report that the management of colon diseases with fecal matter originated in India, a thousand years ago, as it is reported to Charaka-samhita, who in his Uttara-Tantra, describes it. Fact of which there are still many doubts [2]. The procedure was described in the traditional Chinese medicine book Ben Cao Gang Mu, as “yellow soup” [3].
In the seventeenth century, the Italian doctor Girolamo Fabrizi d’Acquapendente, who had Williams Harvey as his most famous student, determined Transplantation as Transfaunation; word from English “Transfaunation”: Transfer part (or all) of the symbiotic flora of the digestive tract [4]. The ingestion of fresh hot dromedary feces was suggested by Bedouins to German soldiers with dysentery, in World War II [5]. In Eiseman B, et al [5] a surgeon from Colorado, United States of America, treated patients with pseudo-membranous colitis, with good results [6]. The bacterium called Clostridium difficile, which generates pseudomembranous colitis, was isolated in 1930, although it was described until 1978 as the causative agent of this disease. That year it was isolated from the stool of a patient receiving Clindamycin [7]. These events are of enormous importance, since FMT is mainly directed at the management of recurrent infection by Clostridiodes difficile.
This represents an annual expenditure of approximately US $4.8 billion for acute care facilities. The epidemiological burden of the disease in 2011 in the United States of America included 434,000 infections and 29,000 deaths [8]. A look at Fecal Microbiota Transplantation for Recurrent C. difficile Infection (RCDI). The Intestinal Microbiota (IM) has enormous benefits in RCDI, which is done through immune processes such as: suppression by antimicrobial peptides, bile acidmediated inhibition of spore germination and vegetative growth, as well as resistance to colonization [9-11]. C. difficile recurs in approximately 10 to 25% of patients treated with antibiotics and this is where we require the use of FMT, to correct the colonization problem. This occurs in about 90% of cases [12]. Now, we will talk about IM, as the subject of C. difficile management has been widely addressed with success. Is the microbiota considered to be? According to the FDA: it is a drug [13]; The European consensus conference on FMT in clinical practice calls it: Tissue to be transplanted [14]. It has also been referred to as: organ or tissue composed of communities [15]. The human fecal microbiota is dominated by a small number of persistent species. Why the insistence on pointing out different denominations? We consider that it is important, since when regulating the procedure, it will be necessary to determine a unique name, in order to advance in the different discussions that are presented, as well as to determine the exact name. What is the procedure called? It has been called Fecal Microbiota Transplantation [16], Stool transplantation; Fecal bacteriotherapy [17], Infusion of stool. Instillation of processed feces. Fecal suspension infusion [18] and Intestinal Microbiota Transplantation (IMT) [19].
Intestinal microbiota
Community of living organisms, resident in the intestine [20], consisting mainly of Firmicutis and Bacteroidetes, including other less abundant species, such as: Actinobacteria, Proteobacteria, Verrucomicrobias, Fusobacteria and Cyanobacteria [21]. No human gut microbiota is the same; the composition depends on many events that occur in childhood, such as: the environment, type of delivery, diet, weaning, as well as the use of antibiotics and others [22].
Dysbiosis
Any change in the composition of the resident commensal microorganisms, relative to the community found in healthy individuals [23]. The altered intestinal bacterial composition (dysbiosis) has been associated with the pathogenesis of many inflammatory diseases and infections, among them we have: Clostridiodes difficile infection, Irritable Bowel Syndrome, Metabolic Syndrome, Inflammatory Bowel Disease, Colorectal Cancer, Multiple Sclerosis, Autism Spectrum Disorder, Obesity, Diabetes Mellitus types 1 and 2, etc. [24]. Therefore, the main objective when these processes occur is to tend to Rebiosis (Return the microbial community to a healthy state) [25].
Methodology
It has to do with: the patient; the donor, the laboratory, and the procedure.
The patient
To carry out the IMT, we do not evaluate sex or age, only that the patients have more than 5 years of the disease, with minimal response to treatment and that the family nucleus looks for another alternative, that offers improvement and are willing to continue some therapy in the medium term, if necessary; Fundamentally: exercises according to age and conditions, prebiotics or symbiotics, and if possible a low-carbohydrate ketogenic diet, as well as Postbiotics and Paraprobiotics. Various publications are provided to all patients so that they understand the procedure and ask all the questions. In the case of children, the information is provided to the responsible family member. We do not force decisions. When the responsible family member and the patient agree, they sign two Letters of informed consent: Endoscopy and Anesthesiology. The Preoperations should not be older than 30 days. Donor Under 25 years old, non-diabetic, obese, without Gastrointestinal surgery and ingested antibiotics or anti-inflammatories in the last 6 months. A complete medical history is performed, including a search for: Irritable Bowel Syndrome, inflammatory bowel disease, colon polyps, and consider that the donor exercise. “The 8-year-old is the best donor,” although there is enormous difficulty in taking blood tests. If you are a potentially healthy donor, request:
Blood
Polymerase chain reaction (PCR). Clostridiodes difficile
Hepatitis A: immunoglobulin (lgM) and (IgG)
Hepatitis B: Surface Antigen (HBsAg)
Hepatitis C: Antibodies.
Human Immunodeficiency Virus (HIV) type 1 and 2 antibodies
(ELISA) Treponema pallidus: rapid plasma reagin test (RPR; if
applicable Positive)
Anti-Cytomegalovirus (IgG) Antibodies
Epstein-Barr Antibodies (IgG)
Naso-Pharyngeal
Polymerase Chain Reaction (PCR). SARS-CoV-2 or IgG antibodies
against SARS-CoV-2, depending on the stage.
Feces
Coproparasitoscopic, in series of 3.
Salmonella, Shigella and Campylobacter stool culture.
Helicobacter pyori antigen.
Rotavirus and Adenovirus antigen.
Vancomycin resistant enterococci.
Syaphylococcus methicillin resistant.
Carbapenem-resistant Enterobacteriaceae: screening culture.
Add those that are determined in the comprehensive Clinical
History.
Donors with positive tests are reported confidentially and
referred for treatment, as appropriate. Blood tests and cultures
are performed every three months; if they are normal, they will
continue as donors.
Clinical exclusion criteria for the donor
History of IBS, IBD, diarrhea, or chronic constipation. Connective tissue diseases. Atopias (eczema, asthma, eosinophilic pathologies of the gastrointestinal system). History of gastrointestinal malignancy. Immunosuppressive drugs. Treatment against neoplasms. Obesity (BMI> 30), type 1 diabetes mellitus. Type 2 diabetes mellitus. Metabolic syndrome, fibromyalgia or chronic fatigue syndrome. AIDS, Hepatitis B and C virus infection or risk of transmission in the last 12 months. Have been in prison; use illicit drugs, be an individual at high sexual risk, have tattoos or piercings, have traveled in the last 6 months to endemic countries with diarrheal diseases or high risk of traveler’s diarrhea. Present with contagious disease or Creutzfel-Jakob disease (Figure 1).
Figure 1:Intestinal microbiota transplantation.
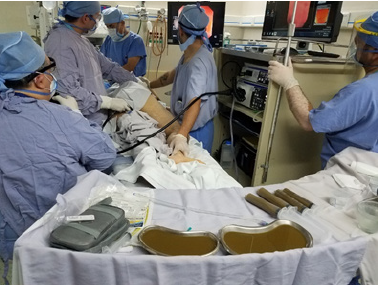
Who can be a donor?
Friends or Relatives. However, anyone can donate stool as long as it is duly studied and has satisfied the requirements by answering a questionnaire similar to the one used for blood donation (Official Mexican Standard NOM-253-SSA1-2012). Next generation sequencing can be used to determine donor eubiosis.
The laboratory
He must be certified and carry out the studies himself.
Microbiota administered by jejunum
The patient is given (6 hours before transplantation) evacuating enema with 45 milliliters of glycerol and two loperamide tablets of 2 milligrams if the patient is not constipated. In a laminar flow hood, 300 to 500grams of feces are mixed (8 hours before transplantation) with 700 to 500 milliliters of saline solution, in order to complete one liter. We homogenize, in a blender. We filter the mixture through sterile gauze (3 layers) and place 1000 milliliters of aliquot in a sterile plastic bottle, covering at the end with a rubber stopper. To extract the gas produced, we place a hypodermic needle number 18 inside the cap for 10 seconds. If the aliquot is not used immediately, it can be refrigerated “Do not freeze”. The bottle should remain in an upright position. This mixture is the microbiota (Table 1 & 2).
Table 1: Conditions involved in the FMT.
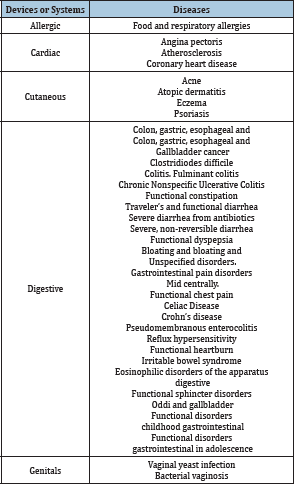
Table 2: Conditions involved in the FMT.
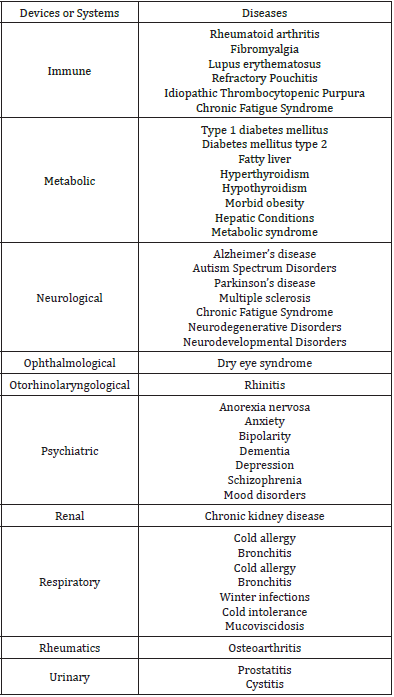
Under general intravenous anesthesia and semi-fowler position, video endoscopy is performed evaluating the esophagus, stomach and duodenum. A biopsy for H. pylori is usually taken at the antrum. In patients with suspected malignancy or a precise indication, biopsies are taken and sent for histopathological study.
The balloon enteroscope is slid up to two centimeters after the Treitz angle. 500 milliliters of microbiota are administered to the jejunum through the tube attached to the balloon enteroscope. At the end of the administration of the microbiota, the transplant will conclude. A 2 milligram loperamide tablet is given when the individual regains consciousness.
Microbiota for colonic administration.
The patient is prepared the colon at least 24 hours in advance based on a liquid diet without dairy or nectars, is administered laxative type Polyethylene glycol (Nulytely) or solution based on dibasic sodium phosphate and monophasic sodium phosphate, 12 hours, before the study. In a laminar flow hood, we mix 300 to 500 grams of feces with 700 to 500 milliliters of saline solution 8 hours before transplantation, to complete one liter. The rest of the preparation is the same as for jejunal administration.
Day of the IMT
The patient must be fasting for 8hours, arrive with an adult companion and sign the informed consent sheets (Anesthesiology and Endoscopy). The administration of Loperamide 2 tablets (antidiarrheal in a single dose) is essential before the transplant is performed, this, in order to minimize intestinal movements, which leads to prevent the patient from evacuating immediately after transplantation and loss part of it. The Endoscopist must be an expert. The procedure is performed under intravenous general anesthesia administered by an expert specialist anesthesiologist.
The totally liquid microbiota is introduced into the gastrointestinal tract by colonoscopy, 1000ml are deposited in the colon, distributed throughout its entire trajectory: ascending (350ml), transverse (300ml) and descending (350ml), After the transplant, the patient is left to rest for a minimum of one hour, trying to avoid, likewise, that when moving, the desire to evacuate is triggered. Foley catheter number 18 is usually placed in the rectum for 2 hours, with a balloon number 30, to avoid the loss of microbiota. The probe is withdrawn as soon as the individual regains consciousness. The frozen microbiota approach to IMT has a number of advantages by eliminating fecal odor, reducing the instilled volume and opening the possibility of future donor stool banks, by guaranteeing the preservation of the microbiota.
Different substances have been used as diluents, 0.9% saline being the most widely used. It is followed by water, and less frequently milk or yogurt [26]. The volume of the diluent varies from 500 to 700ml. IMT solutions made with water have had higher cure rates in recurrent C. difficile infection than those using saline (98.5% vs. 86%). However, the risk of recurrence of C. difficile infection can be up to 2 times higher when using water than when using saline (8% vs. 3%). Other diluents, like milk, achieve resolution rates of up to 94%. To prepare the solution, the faeces and the diluent must be homogenized in a blender until they reach a liquid consistency and then the solution obtained must be filtered to eliminate the greatest amount of residual products. Depending on the route of administration, the solution can be deposited in bags for administration via rectal enema or in syringes for administration via nasogastric tube or via colonoscope channel. Colonoscopy allows administration of the suspension throughout the entire colon and terminal ileum. Different ways have been proposed to carry out the administration of the microbiota during colonoscopy, the most commonly used is gradual instillation every 5-10cm. Until 1989, the most used route of administration was the retention enema. The transplant can be administered by nasogastric tube, naso-duodenal tube, jejunoscope, colonoscope, retention enema or capsule. The colonic route being the most effective. Fresh samples should not be exposed to temperatures above 20 °C, and refrigeration to 4 °C may be a safe solution [27]. Cryopreservation can be considered as the gold standard [28]. The syringes prepared to install the Microbiota are observed and the Foley catheter to be applied. Below are three tables with conditions that respond to transplantation. How many transplants have been performed? To date, there are hundreds of cases carried out. Only Pubmed concentrates 2000 articles on the subject. We have series of more than 500 patients studied, such as those of Kassam Z et al. [28]; that of Cui B et al. [29], those of van Beurden YH et al. [30] with more than 200 cases [30], and Rossen NG et al. [31]. As well as various authors who manage multiple patients [32,33]. In all of them, the potential of IMT is manifest and it achieves cures in C. difficile close to 95% and in other diseases the result varies, but there is always improvement, except for honorable negativities.
How long does the Microbiota remain stable, once constituted?
Without a doubt, the first thing that must be taken into account in the answer to the previous question are the processes experienced in childhood [34]. As soon as IM composition is established, it is relatively stable throughout adult life. It can, however, be altered as a result of the type of birth, lifestyle, bacterial infections, surgeries, antibiotics, and change in diet, in the long term [35,36]. It has been pointed out that if the microbiota is kept at room temperature in aluminum bags, with plastic, hermetic to humidity and air, they can be reconstituted to their original volume, with reduced ultra-pure water and the viability is usually up to 30% [37]. In astronauts, IM showed conserved dynamics. They were in space for 2 years. This fact will undoubtedly help the understanding of the Microbiome [38]. Likewise, they have been found up to four and a half years after successful FMT, verified at the molecular level [39]. Some authors have stated that more studies are required to determine the time that the transplanted Gut Microbiota continues to be efficient [40]. For all the above, the use of probiotics, prebotics, symbiotics, low carbohydrate ketogenic diet, as well as postbiotics and paraprobiotics, Bactrian consortium transplantation, phage therapy and therapeutic complement should be evaluated [41]. In conclusion, FMT is a very effective treatment for CDI recurrence, with long-term benefits over the use of antibiotics [42].
Antibiotics before transplant?
We consider that dysbiosis is strongly linked to long-term antibiotic therapy; it can be determined that these drugs would not be helpful in pre-transplantation. However, as the answer remains controversial, we had to dig deeper [43]. In favor of the non-use of antibiotics, it is said that they reduce the goodness of the transplant [44], as well as affect the quality of the Microbiota [45]. There is a meta-analysis that comments otherwise [46]. It is even noted that they improve the diversity of Bacteroidetes [47]. Metagenomic and metatrascriptomic analysis. Within these analyzes necessary to measure the impact of transplantation, the recovery of intact RNA and DNA is a significant step in these studies and for this, a very good stool storage is decisive, and thus not damaging nucleic acids [48]. For optimal preservation, stool samples should be kept at room temperature and brought to the laboratory within 24hours of collection or immediately stored at -20 °C in a freezer domestic and then transported in a freezer pack to ensure they do not thaw at any time [49].
Complications
In the various countries where Intestinal Microbiota Transplants are performed, complications have appeared, but these are usually mild such as: diarrhea, fever, bloating and abdominal pain, as well as rashes, in immunocompromised patients, which are reversible [50]. Long-term impact. IMT usually generates profound changes in the recipient’s Gut Microbiota, which are usually beneficial, in a safe procedure [51]. More studies must be done to determine its longevity.
The future
There are many aspects that will be addressed in both the mid-term and late future. We will be able to try to elucidate the ambiguities and understand the complexity of the microorganismhuman being relationships; determining the existing profiles in the various Gut Microbiota, to assess the current status, as well as the important intestinal function [52]. We will seek to determine the impact of the microbiota on both health and disease [53]. Genomic knowledge of the microbiota in relation to metabolic development will increase [54]. New specific probiotic strains will be discovered, capable of improving intestinal processes [55]. Microbiota transplantation will be an inevitable complement to conventional treatments [56]. Some of the following questions may be answered: Is Transplantation better than traditional treatments? How often should the transplant be repeated? How safe is the transplant? Will there be long-term, unexpected side effects? [57-59]. Lastly, it will be determined whether Fecal Microbiota Transplantation is useful in SAR-CoV-2 infections.
Conflicts of Interest
The authors declare that do NOT have affiliation or participation in organizations with financial interests.
Ethical Approval
This report does not contain any study with human or animal subjects carried out by the authors.
Informed Consent
The authors obtained informed written consent from the patients, in order to develop this article.
References
- Zhang F, Luo W, Shi Y, Fan Z, Ji G (2012) Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol 107(11): 1755.
- Vishwakarma R, Goswami PK (2013) A review through Charaka Uttara-Tantra. Ayu 34(1): 17-20.
- Borody T, Warren EF, Leis SM, Surace R (2004) Bacteriotherapy using fecal flora: Toying with human motions. J Clin Gastroenterol 38(6):475-83·
- Soo WT, Bryant RV, Costello SP (2004) Faecal microbiota transplantation: Indications, evidence and safety. Aust Prescr 43(2): 36-38.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ (1958) Fecal enema as an adjunct in the treatment of pseudomembranous Enterocolitis. Surgery 44(5): 854-859.
- Meyer SL, Espinoza AR, Quera PR (2014) Clostridium difficile infection: Epidemiology, diagnosis and therapeutic strategies. Las Condes Clinical Medical Journal 25(3): 473-484.
- Lessa FC, Mu Y, Bamberg VM, Beldavs ZG, Dumyati GK, Dun JR, et al. (2015) Burden of Clostridium difficile infection in the United States. N Engl J Med 372(24): 2369-2370.
- Ostaff MJ, Stange EF, Wehkamp J (2013) Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med 5(10): 1465-1483.
- Thanissery R, Winston JA, Theriot CM (2017) Inhibition of spore germination, Growth, and toxin activity of clinically relevant difficile strains by gut microbiota derived secondary bile acids. Anaerobe 45: 86-100.
- Fehervari Z (2019) Mechanisms of colonization resistance. Natureresearch, pp. S17-S18.
- Borody TJ, Ramrakha S (2020) Fecal microbiota transplantation for treatment of recurrent Clostridioides (formerly Clostridium) difficile In: Rutgeerts P, Barol EL (Edn.),.
- (2019) FDA In Brief: FDA warns about potential risk of serious infections caused By multi-drug resistant organisms related to the investigational use of fecal microbiota for transplantation.
- Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, et al. (2017) European consensus conference on faecal microbiota Transplantation in clinical practice. BMJ Journal Gut 66(4): 569-580.
- Bartman C, Chong AS, Alegre ML (2015) The influence of the microbiota on the immune response to transplantation. Curr Opin Org Transplant 20(1): 1-7.
- Kim KO, Gluck M (2019) Fecal microbiota transplantation: An update on clinical practice. Clin Endosc 52(2): 137-143.
- Gupta S, Allen-Vercoe E, Petrof EO (2016) Fecal microbiota transplantation: In Perspective. Therap Adv Gastroenterol 9(2): 229-239.
- Choi HH, Cho YS (2016) Fecal microbiota transplantation: Current applications, effectiveness, and future perspectives. Clin Endosc 49(3): 257-265.
- Bajai JS, Khoruts A (2020) Microbiota changes and intestinal microbiota transplantation in liver disease and cirrhosis. Journal of Hepatology 72(5): 1003-1027.
- Sebastián Domingo JJ, Sánchez-Sánchez C (2018) From the intestinal flora to the microbiome. Rev Esp Enferm Dig 110(1): 51-56.
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAB, Gasbarrini, et al. (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7(1): 14.
- McBurney MI, Davis C, Fraser CM, Schneeman BO, Huttenhower C, et al. (2019) Establishing what constitutes a healthy human gut microbiome: State of the science, regulatory considerations, and future directions. The Journal of Nutrition 149(11): 1882-1895.
- Petersen C, Round JL (2014) Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 16(7): 1024-1033.
- Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26: 26191.
- Franke ML (2014) Symbiosis, dysbiosis, and rebiosis-the value of metaproteomics in human microbiome monitoring. Proteomics 15(5-6): 1142-1151.
- Burz SD, Abraham AL, Fonseca F, David O, Chapron A, et al. (2014) A Guide for Ex Vivo handling and storage of stool samples intended for fecal microbiota transplantation. Sci Rep 9(1): 8897.
- Aroniadis OC, Brandt LJ (2013) Fecal microbiota transplantation: Past, present And future. Curr Opin Gastoenterol 29(1): 79-84.
- Vandeputte D, Tito RY, Vanleeuwen R, Falony G, Raes J (2017) Practical considerations for large-scale gut microbiome studies. FEMS Microbiology Reviews 41(Supp 1): S154-S167.
- Kassam Z, Lee C, Yuan Y, Hunt R (2013) Fecal microbiota transplantation for Clostridium difficile infection: Systematic review and Meta-analysis. Am J Gastroenterol 108(4): 500-508.
- Cui B, Li P, Xu L, Peng Z, Xiang J, et al. (2016) Step-up Fecal Microbiota Transplantation (FMT) strategy. Gut Microbes 7(4): 323-328.
- Van Beurden YH, de Groot PF, van Nood E, Nieuwdorp M, Keller JJ, et al. (2017) Complications, effectiveness, and long term follow-up of fecal Microbiota transfer by nasoduodenal tube for treatment of recurrent Clostridium difficile United European Gastroenteerol J 5(6): 868-879.
- Rossen NG, MacDonald JK, de Vries EM, D'Haens GR, de Vos WM, et al. (2015) Fecal microbiota transplantation as novel therapy in Gastroenterology: A systematic review. Worl J Gastroenterol 21(17): 5359-5371.
- Hamilton M, Weingarden A, Unno T, Khoruts A, Sadowsky M (2013) High-throughput DNA sequence analysis reveals stable engraftment of gut Microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes 4(2): 125-135.
- Rohlke F, Surawicz CM, Stollman N (2010) Fecal flora reconstitution for recurrent Clostridium difficile infection: Results and methodology. J Clin Gastroenterol 44(8): 567-570.
- Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, et al. (2015) The Composition of the gut microbiota throughout life, with an emphasis on Early life. Microb Ecol Health Dis 26: 26050.
- Gagliardi A, Totino V, Cacciotti F, Lebba V, Neroni B, et al. (2018) Rebuilding the gut microbiota ecosystem. Int J Environ Res Public Health 15(8): 1679.
- Dickson L (2019) Stability and individual of adult microbiota natureresearch. Milestones.
- Broecker F, Klumpp J, Schuppler M, Russo G, Biedermann L, et al. (2016) Long-term changes of bacterial and viral compositions in the intestine of a recovered Clostridium difficile patient after fecal microbiota Transplantation. Cold Spring Harb Mol Case Stud 2(1): a000448.
- Turroni S, Rampelli S, Biagi E, Consolandi C, Severgnini M, et al. (2017) Temporal dynamics of the gut microbiota in people sharing a confined Environment, a 520-day land-based spatial simulation, MARS500. Microbiome 5: 39.
- Agrawal M, Aroniadis OC, Brandt LJ, Kelly CR (2015) The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated clostridium difficile infection in 146 elderly individuals. Journal of clinical gastroenterology 50(5): 403-407.
- Paoli A, Mancin L, Bianco A, Thomas E, Mota JF, et al. (2019) Ketogenic diet and microbiota: Friends or enemies? Genes (Basel) 10(7): 534.
- Nowak A, Hedenstierna M, Ursing J, Lidman C, Nowak P (2019) Efficacy of routine fecal microbiota transplantation for treatment of recurrent Clostridium difficile Infection: A Retrospective Cohort Study. Int J Microbiol: 7395127.
- Gagliardi A, Totino V, Cacciotti F, Lebba V, Neroni B, et al. (2018) Rebuilding the gut microbiota ecosystem. Int J Environ Res Public Health 15(8): 1679.
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knigth R (2012) Diversity, stability and resilience of the human gut microbiota. Nature. Volume 489(7415): 220-230.
- Dudek-Wicher RK, Junka A, Bartoszewicz M (2018) The influence of antibiotics And dietary components on gut microbiota. Prz Gastroentrol 13(2): 85-92.
- Rokkas T, Gisbert JP, Gasbarrini A, Hold GL, Tilg H, et al. (2019) A network meta-analysis of randomized controlled trials exploring the role of Fecal microbiota transplantation in recurrent Clostridium difficile United European Gastroenterol J 7(8): 1051-1063.
- Hertz FB, Budding AE, van der Lugt-Degen M, Savelkoul PH, Løbner-Olesen A, et al. (2020) Effects of antibiotics on the intestinal microbiota of mice. Antibiotics (Basel) 9(4): 191.
- Cardona S, Eck A, Cassellas M, Gallart M, Alastrue C, et al. (2012) Storage conditions of intestinal microbiota matter in metagenomic analysis. BMC Microbiology 12: 158.
- Tabibian JH, Kenderian SS (2017) The microbiome and immune regulation after transplantation. Transplantation 101(1): 56-62.
- Goloshchapov OV, Olekhnovich EI, Sidorenko SV, Moiseev IS, Kucher MA, et al. (2019) Long-term impact of fecal transplantation in healthy Volunteers. BMC Microbiol 19(1): 312.
- Zatorski H, Fichna J (2014) That is the future of the gut microbiota-related treatment? Toward modulation of microbiota in Preventive and therapeutic medicine. Front Med (Lausanne) 1: 19.
- Kitazawa H, Alvarez S, Suvorov A, Melnikov V, Villena J, et al. (2015) Recent advances and future perspective in microbiota and probiotics. Biomed Res Int, p. 275631
- Malla MA, Dubey A, Kumar A, Yadav S, Hashem A, et al. (2018) Exploring the human microbiome: The potential future role of next-generation sequencing in disease diagnosis and treatment. Front Immunol 9: 2868.
- Day LLJ, Harper AJ, Woods RM, Davies OG, Heaney LM (2019) Probiotics: Current landscape and future horizons. Future Sci OA 5(4): FSO391.
- Rosenbaum JT (2019) Just another crappy commentary: The future of fecal Microbiota transplantation. Expert Review of Clinical Immunology 15(10): 987-989.
- Rao K, Young VB (2015) Fecal microbiota transplantation for the management of Clostridium difficile Infect Dis Clin North Am 29(1): 109-122.
- Hota SS, Poutanen SM (2018) Is a single fecal microbiota transplant a promising treatment for recurrent Clostridium difficile infection? Open Forum Infectious Diseases 5(3): 045.
- Rodríguez de Santiago E, García de Paredes AG, Aracil CF, Castro LA, San Román AL (2015) Fecal microbiota transplantation: Indications, methodology and future prospects REV. Argent Coloproct 26(4): 225-234.
- Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, Ng SC, et al. (2020) Reorganisation of faecal microbiota transplant services during the COVID- 19 pandemic. Gut 69(9): 1555-1563.
- Álvaro Zamudio T, Silverio AL (2020) Is Dysbiosis the Source of the problem in SARS-CoV-2 infection? EC Microbiology Special Issue SI. 02: 20-22.
© 2021 Álvaro Zamudio Tiburcio. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






