- Submissions

Full Text
Gastroenterology Medicine & Research
Evaluation of 68Ga-labeled TPP-1: A Targeted PDL1 Probe for PET Imaging of Colorectal Cancer
Xiaojiao Wang1, Beibei Zhang2, Xiao Li1* and Danni Li1*
1Department of Nuclear Medicine, Shanghai Changhai Hospital, Shanghai, China
2Shenzhen Second People’s Hospital, Shenzhen, China
*Corresponding author:Danni Li and Xiao Li, Department of Nuclear Medicine, Shanghai Changhai Hospital, Shanghai, China
Submission: February 05, 2021;Published: February 15, 2021

ISSN 2637-7632Volume5 Issue4
Abstract
Although PD-1/PD-L1 immuno-blocking therapy (IBT) has improved the prognosis of patients with gastrointestinal tumors to some extent, not all patients respond to IBT. Recent studies have shown that PD-L1 antigen expression in tumors is prognostic marker of response to PD-1/PD-L1 IBT. Here, 68Ga-TPP-1 was developed for specific and non-invasive imaging of PD-L1 expression in mouse models of colorectal cancer. Positron emission tomography (PET) and immunohistochemistry (IHC) results confirmed the highly specific targeting of 68Ga-TPP-1 in PD-L1 expressing colorectal cancer mice.
Keywords: Immuno-PET imaging; Colorectal cancer; PD-L1; Ga-68
Introduction
Colorectal cancer is a common clinical malignancy of the digestive system. In recent years, with the improvement of living standards and changes in dietary habits, the incidence and death rate of colorectal cancer has been on the rise [1]. At present, effective treatments for colorectal cancer include surgery, chemotherapy and radiotherapy, but most lesions have already metastasized at the time point of being detected, resulting in a high death rate. The development of immunotherapeutic agents targeting PD-1/PD-L1 has affected the treatment strategy for many digestive system tumors due to their high efficacy and low toxicity compared to conventional treatments [2,3]. Unfortunately, although immuno-blocking therapy (IBT) targeting the PD-1/PD-L1 pathway has shown impressive clinical results, only a small number of patients have been benefited [4].
Multiple studies have shown that PD-L1 antigen expression is a prognostic marker of response to PD-1/PD-L1 IBT. Recently, positron emission tomography (PET) imaging with radiolabeled anti-PD-L1 antibodies has shown vital advantages over immunohistochemistry in evaluating PD-L1 expression [5]. PD-1/PD-L1-targeted nuclear imaging enables the quantification of primary and metastatic lesions as well as systemic PD-1/PD-L1 expression by replacing invasive tests such as puncture, providing a more accurate screening method for patients on PD-1/PD-L1 immunotherapy. However, the disadvantage of nuclide-labeled intact monoclonal antibodies is of the need to use long half-life nuclear and prolong the time required for imaging. Herein, small molecule peptide probes are ideal as a PD-L1 PET imaging agent as they are more penetrating to tumors and can be rapidly up taken by tumors and cleared from the blood and background tissue, enabling the same-day imaging by one injection. TPP-1 is a PD-L1-targeting and clinically translatable peptide that has demonstrated its potential for rapid and highly specific binding to PD-L1 in several preclinical studies [6,7]. In this study, the tracer 68Ga-TPP-1 showed good application in PD-L1 expression imaging in MC38 xenograft tumors and had promising applications in guiding immunotherapy.
Methods
For the synthesis of 68Ga-TPP-1, TPP-1-DOTA (50μg, in 0.25M sodium acetate, 1mL) was mixed with fresh 68Ga3+(in 0.05M HCl, 4mL). The reaction mixture was incubated at 100℃ for 10min and purified using a sep-pak C-18 cartridge with 50% ethanol as the eluent. For animal studies, the right upper limb of three combined immunodeficient mice (line name: NOD-Prkdcscid Il2rgem1/Smoc) were injected with murine colorectal carcinoma cell MC38 that was of high PD-L1 expression [8]. PD-L1 PET imaging was performed when tumor length reached approximately 7-8mm. All experiments for animal research were conducted in accordance with the principles laid out by the ethical committee of Shanghai Changhai hospital. For PET imaging studies, 3.7MBq 68Ga-TPP-1 was injected into the tail veins of a mouse bearing colorectal cancer (N=3), the blocking group was pre-injected with the 20-fold molar excess of unlabeled TPP-1 one hour in advance. PET imaging was performed at 30, 60 and 90min post-injection. Regions of interest (ROI) were drawn over tumors and contralateral muscles. The data analysis of PET images was performed with INVEON software (Siemens). Immunohistochemical analysis of tumor tissues was performed for PD-L1 antigen immediately after PET imaging.
Results
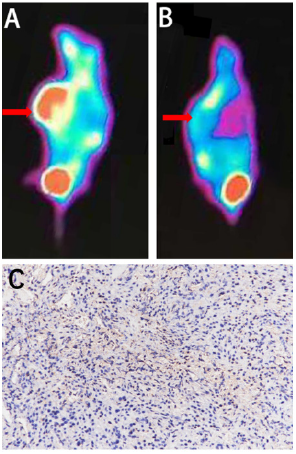
The radio-HPLC results showed the radiochemical purity of 68Ga-TPP-1 was approximately 99% after purification. 68Ga-TPP-1 was stable (>95%) in 0.01M PBS at 37℃ for 6h, as well as displayed acceptable stability (> 90%) in 1% mouse serum for 4 hours. PET imaging showed that the tumor was of highly uptake of 68Ga-TPP-1, and the physiological uptake in the kidney and bladder were detected. Tumor uptake of 68Ga-TPP-1 was detected at 30 min post injection, and the SUVmax of the tumor and contralateral muscles was 0.95±0.06 and 0.42±0.11 at 60min post-injection, respectively. The targeting uptake at the tumor increased gradually during the first-hour post injection, and the tumor can be clearly seen at 90min post injection. The tumor-to-muscle (T/M) uptake ratio peaked at 2.33±0.55 at 60min post injection. Uptake of 68Ga-TPP-1 in tumor was significantly (p<0.05) blocked when compared to non-blocked group, where the SUVmax of the tumor was 0.33 (Figure 1). This revealed that, in addition to its physiological distribution in the kidney and bladder, 68Ga-TPP-1 has better specific binding to PD-L1 high expression colorectal cancer. Based on the immunohistochemistry results of tumors tissues, PD-L1 antigens were expressed in MC38 tumor massively where the brown-yellow area stands for positive expression (Figure 1).
Figure 1: A typical 68Ga-TPP-1 PET images of the tumor mouse model and corresponding immunohistochemistry
of PD-L1.
A. PET imaging studies of MC38 cell tumor-bearing model at 60 min post injection of 68Ga-TPP-1.
B. PET imaging of blocking group mice model at 60 min post injection of 68Ga-TPP-1, and the arrow pointed
to the location of xenograft.
C. Immunohistochemistry confirmed the high PD-L1 expression in tumor tissue (original magnification ×200).
Discussion
In contrast to traditional treatments for gastrointestinal tumors, immunotherapy focuses on improving the microenvironment in and around the tumor and enhancing the body's own immune function, resulting in the inhibition of tumor cell growth. However, the low efficiency and high cost of treatment restrict the application of IBT, and the current methods of screening patients for benefit, such as IHC and dMMR, do not accurately predict the efficacy of PD-1/PD-L1 IBT, which remains a serious issue pending to be solved. The clinical significance of immune-related molecular imaging is demonstrated in guiding treatment and predicting therapeutic efficacy. Functional imaging in nuclear medicine has been shown to be useful in assessing PD-1/PD-L1 expression in a variety of tumor types. Currently, three PD-1/PD-L1 targeting probes have been successfully applied in translational clinical studies [5,9]. For example, Nature Medicine reported that it is possible to image non-small cell lung cancer, advanced bladder cancer and triple negative breast cancer with 89Zr-Atezolizumab PET, demonstrating heterogeneous variation between lesions, patients and tumor types as well as laying the foundation for establishing clinical treatment options. In terms of PD-1/PD-L1 molecular imaging, the targeting ability and metabolic pathway of the probes play a key role. Objective issues such as poor penetration of intact antibodies in solid tumor tissue, low stability, restricted routes of drug delivery and difficulty to control pharmacokinetics limit their use in clinics [10].
In this situation, the advantages of small molecule PD-L1 inhibitors with a high degree of specificity are highlighted. Compared to antibodies, peptide-based PD-1/PD-L1 inhibitors have favorable biocompatibility and ease of synthesis, offering an advantage in avoiding accumulation in non-target organs. Therefore, the development of low molecular weight probes as 68Ga-TPP-1 that specifically bind to PD-L1 with high affinity will be an important direction for future research. This will enable highly specific detection of PD-1/PD-L1 expression in patients while performing SPECT or PET imaging in a short time after probe injection and will provide a more convenient imaging method for the clinical application of tumor immunotherapy.
References
- Siegel R, Miller K, Jemal A (2020) Cancer statistics. CA A Cancer J Clin 70(1): 7-30.
- Yagi T, Baba Y, Ishimoto T, Masaaki I, Yuji M, et al. (2019) PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann Surg 269(3): 471-478.
- Valentini A, Di Pinto F, Cariola F, Vito G, Gianluigi G, et al. (2018) PD-L1 expression in colorectal cancer defines three subsets of tumor immune microenvironments. Oncotarget 9(9): 8584-8596.
- Geng Q, Jiao P, Jin P, Gaoxing S, Jinlong D, et al. (2017) PD-1/PD-L1 Inhibitors for immuno-oncology: From antibodies to small molecules. Curr Pharm Des 23(39): 6033-6041.
- Bensch F, Van der Veen EL, Lub-de Hooge MN, Annelies J, Ronald B, et al. (2018) 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 24(12): 1852-1858.
- Li C, Zhang N, Zhou J, Chen D, Yaqing J, et al. (2018) Peptide blocking of PD-1/PD-L1 interaction for cancer immunotherapy. Cancer Immunol Res 6(2): 178-188.
- Hu K, Hanyu M, Lin X, Yiding Z, Lin X, et al. (2019) Developing native peptide-based radiotracers for PD-L1 PET imaging and improving imaging contrast by pegylation. Chem Commun 55(29): 4162-4165.
- Juneja V, Mcguire KA, Manguso RT, Martin W, Natalie C, et al. (2017) PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 214(4): 895-904.
- Niemeijer A, Leung D, Huisman MC, Bahce I, Hoekstra O, et al. (2018) Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun 9(1): 4664.
- Li D, Wang C, Zhang DK, Ye Peng, Shengnan R, et al. (2018) Preliminary application of 125I-nivolumab to detect PD-1 expression in colon cancer via SPECT. J Radioanal Nucl Chem 318: 1237-1242.
© 2021 Danni Li, Xiao Li. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






