- Submissions

Full Text
Gastroenterology Medicine & Research
Comparative Efficacy of Rectal Nonsteroidal Anti-Inflammatory Drugs and Pancreatic Duct Stents in Preventing Pancreatitis After Endoscopic Retrograde Cholangiopancreatography: A Network Analysis
Yin Wang1*, Qian Zhang2 and Fu Cheng Zhang3
1Department of Gastroenterology, The people’s hospital of Bozhou, Ahhui province, China
2Department of pharmacy, The people’s hospital of Bozhou, Ahhui province, China
3Department of Gastroenterology, Shandong Provincial ENT Hospital affiliated to Shandong University, Jinan, Shandong province, China
*Corresponding author:Yin Wang, Department of Gastroenterology, The people’s hospital of Bozhou, Ahhui province China
Submission: February 24, 2020;Published: March 10, 2020

ISSN 2637-7632Volume4 Issue4
Abstract
Background/Aims: Some meta-analyses suggest that pancreatic duct stents (PDS) placement and rectal nonsteroidal anti-inflammatory drugs (NSAIDs) can prevent post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP), but which is favorable is unclear. Thus, we conducted network analyses to compare treatment efficacies.
Materials and Methods: The Cochrane controlled trial registry, PubMed, China Biology Medicine disc (CBM disc) and Embase were reviewed, focusing on the randomized controlled trials (RCTs) of rectal NSAID and PDS prophylaxis throughout the PEP prophylaxis process On September 25, 2019. Reviewers extract data independently and randomly. The Cochrane handbook was used as a standard to assess migration risk, and intervention management was implemented in a systematic way. The rationality of the evidence is determined by means of grade evaluation, proposal formulation and evaluation, and the main output result is the effect of intervention.
Results: The RCTS was 32, and the total number of participants involved was 5311. Combined with the results of the standard meta-analysis, it was found that rectal NSAIDs and PDS implants significantly reduced the likelihood of PEP, PDS: OR= 0.34, 95% Confidence interval (CI) 0.24 to 0.49, I2=10.9%, Diclofenac (DIC): OR= 0.31 95% CI 0.16 to 0.59 I2=47.3%; indomethacin (ID): OR= 0.56 95% CI 0.41 to 0.76 I2=33.8%; Naproxen (NAP): OR= 0.38 95% CI 0.19 to 0.78. Network analysis of all trials demonstrated the quality of PDS OR 0.32 95% CI 0.21 to 0.48, DIC: OR 0.30 95% CI 0.18 to 0.49, ID: OR= 0.55 95% CI 0.39 to 0.77, NAP: OR= 0.55 95% CI 0.39 to 0.77. Network analysis ranking showed that PDS placement was the best way for preventing PEP, followed by NAP, ID, and DIC.
Conclusion: This evidence suggests that PDS placement is most effective in reducing PEP risk. However, rectal NSAIDs may be more ideal due to decreased costs.
Keywords: Rectal nonsteroidal anti-inflammatory drugs; Pancreatic duct stents; Post-endoscopic retrograde cholangiopancreatography pancreatitis; Network analysis
Introduction
In the clinical treatment of pancreatic diseases, Endoscopic retrograde cholangiopancreatography (ERCP) has a very wide range of applications, at the same time in this process is usually accompanied by a lot of complication, the Symptoms include PEP, perforation, bleeding, and infection, which belongs to the whole ERCP in the most common and common type of syndrome. The probability of occurrence of PEP pancreatitis generally remains in the range of 3% to 10% [1,2], whereas for high-risk individuals, the probability of developing PEP typically ranges from 15% to 20% [3]. A meta-analysis of 13296 participants indicates that the probability of all patients having PEP is 9.7%, whereas the Incidence of PEP. in high risk participants was 14.7%, most participants had mild acute pancreatitis, total mortality was 0.7% [4,5], the large number of illnesses that result from PEP have a huge impact and cost the entire health care system; according to data provided by USA, the annual costs associated with managing PEP have reached more than $200 million [4].
Drug prophylaxis of PEP has always been a hot topic in clinical research, the optimal drugs for preventing PEP have common characteristics such as efficacy, low side effect profile, high cost-benefit ratio, easy acquisition and easy administration. For the people with the process and high-risk patients may be at higher risk, NSAID for preventing the patient produces PEP has the extremely widespread application value, some meta-analysis [6-8] by pointed out that such as the European association for gastrointestinal endoscopy and Japan GanDanYi surgical association guidelines are referred to such a question, namely for high-risk patients can be treated with NSAIDs [9-13]. There are also a number of meta-analyses that show that PEP can be largely prevented by stenting the pancreas. But the two approaches also raise questions about whether the interventions are theoretically complementary and whether they are synergistic. We need to give further demonstration and research. Network meta-analysis was used to effectively analyze the data. It includes studies on various types of treatment and prevention strategies that show that rectal maids are significantly more effective than pancreatic stents in the prevention of PEP [14], but a few studies have a publishing bias against these findings, non-randomized controlled trials. Thus, the main purpose of this study is to compare the differences between the two through network meta-analysis and system review NSAIDS and stents for preventing PEP, all randomized controlled trials were involved and prevention was performed for the best PEP intervention The RCT-related data were searched comprehensively. In order to eliminate language restrictions, we generally use the following terms: non-steroidal anti-inflammatory drugs, pancreatic*, endoscopic retrograde cholangiopancreatography, ERCP, NSAIDS, stent, random* combined with all content included rectal NSAIDs. We included RCTs that compared rectal NSAIDS or stents to placebo controls or other rectal NSAIDS, used for the prevention of PEP in patients. We analyzed only RCTs because placebo was included as part of the network meta-analysis, for RCT study design, it is mainly used to ensure the rigor and accuracy of the whole study process by determining the deviation and minimizing the performance [15]. Two reviewers, Zhang Qian and wang Yin, reviewed the search results to prevent the reports from being ignored, if there were any disagreements, the third reviewer (Fu-Cheng Zhang) made the final decision.
Materials and Methods
Data extraction
The uniformity of the data extracted for the purpose of lifting, the reviewers, (Yin Wang, Qian Zhang) independently extracted the original data presented in the literature onto a standardized form: country, inclusion criteria, year of publication, intervention time, the first author, Rectal NSAIDs (drug, dose, NO.), control (placebo (PLA)/NO.), NO. of PEP. If necessary, the author of the study was contacted in order to obtain the study data. The conflict of abstract data can be solved by negotiation and reference to the original article.
Ethic committee approval
Because the type of the paper is reviewing whose materials are derived from primitive clinical research from other authors, we do not have Informed Consent necessarily
Risk of bias assessment
The authors mainly used the Cochrane collaboration manual [16] as a reference for independent evaluation of the quality of the literature. The criteria included in the scoring system are selective scaling, ignorance of assessment results, incomplete result data, and ignorance of participants, random sequence generation and other sources of bias. The results diagrams presented were created by using the soft Reman 5.2 (The Nordic Cochrane Center, Denmark).
Statistical analysis
Stata15.0 (StataCorp, Texas) was used in our analysis for standard meta-analysis to achieve direct comparison. Two different types of data were used to study and analyze the likelihood of the onset of PEP, and to calculate the confidence curve and relative risk, and effectors. The heterogeneity between the results of the study were examined using the Q test (test level is a=0.1), and the magnitude of heterogeneity was judged by combining the findings with the I2 test [17]. I2-related studies with volume fractions in the 25% to 50% range had low heterogeneity. At the same time, I2-related studies with volume fractions ranging from 50% to 75% had moderate heterogeneity. Studies of I2 with a volume fraction of more than 75% had higher heterogeneity. If the I2 score is more than 50%, then the model should be constructed by random effect. If not, then a fixed effect model needs to be built for the above situation. Dual-sample test and funnel plot were used to evaluate the deviation. In addition, dual-sample test was used to evaluate the heterogeneity between target objects. If P <0.05, it means that there is a significant difference in the field of statistics. At the same time, the possibility of publication deviation can also be evaluated by means of funnel plot asymmetry. Refer to PRISMA's statement for meta-analysis.
Network meta-analysis
When carrying out data analysis, we mainly used network meta-analysis with the software Stata15.0 and the momenta command. The first step is to build a network of evidence whose main purpose is to compare treatments. By means of 95%Cl and inconsistent factors, the consistency of each closed-loop part was evaluated and analyzed. The consensus is that 95%Cl is 0. If not, then this part of the closed loop will be identified as significantly inconsistent. In this paper, all output indexes are used for data counting. Therefore, RR combination is used to combine the effect, and 95%Cl is used to estimate the interval size. The upper limit of 95%Cl mentioned above is less than 1, while the lower limit is greater than 1, according to the above results, it can be known that there are significant differences between the two, otherwise it would be considered that there was no significant difference in the field of statistics. Results were obtained after a network meta-analysis, and PR Best and SUCRA were used to rank the results. For subgroup analysis, higher SUCRA scores were associated with better therapeutic outcomes. In addition, PR Best represents the probability that the treatment is the best treatment. After obtaining the data results, a recommendation assessment, a development assessment, and a registration assessment [18] are required for each result, and the specific results obtained are displayed in each breakdown. If there is no deviation limitation due to reporting deviation, research treatment, inconsistency, data sparsity, inaccuracy, etc., then the obtained RCT will be considered as high-quality certification. If any of the above occurs, the level of evidence will be classified as moderate or low.
Results
Studies and risk of bias assessment
1348 studies were retrieved, but 71 studies were excluded after duplicates. After the title filter and summary filter are completed, in the constructed screening criteria, the number of constraints that did not meet the requirements was 108, and the number of papers that were eliminated was 1136, 26 studies used non rectal NSAIDS, 20 studies were meta-analysis, 23 studies were non-human experiments and 40 studies were not RCTs. Finally, there were 32 options for the control group, including 31 English studies and 1 Chinese study [19-50] (Figure 1).
Figure 1: Summary of flow diagram.
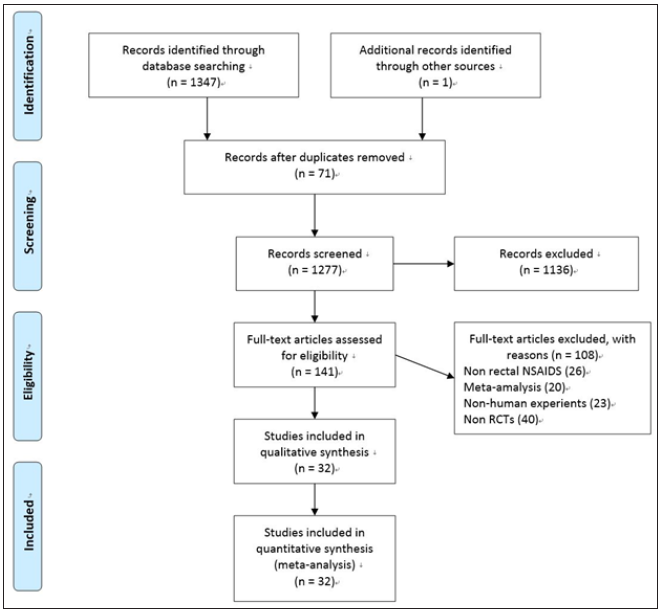
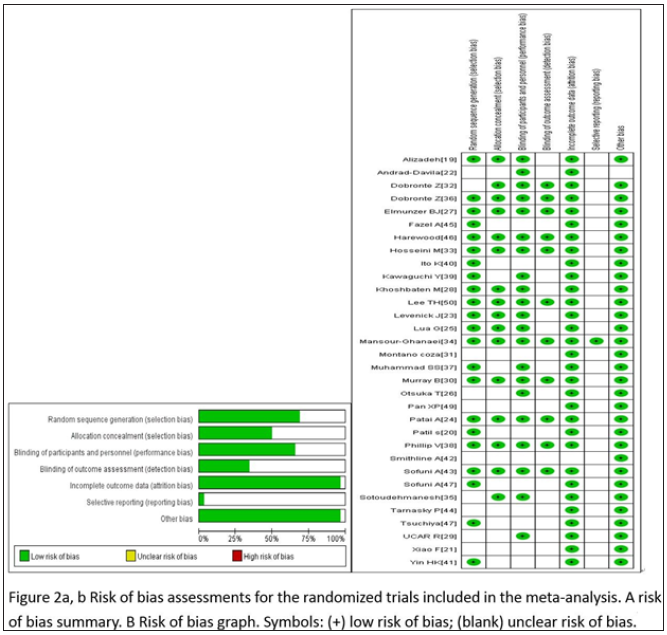
The studies with administration of rectal NSAIDS ranged from 2003 to 2018, whereas studies with stents ranged from 1993 to 2019. The regional distribution was from all over the world, Iran [19,28,33-35], India [20], China [21,41,49], Mexico [22,31], USA [23,27,42,44-46], Hungry [24,32,36], Malaysia [25], Japan [26,39,40,43,47,48], Turkey [29], Scotland [30], Pakistan [37], South Korea [50], European countries [38]. Most of the studies included high-risk patients [20,22,25,27,28,30,39-48,50], the others were non-high risk patients [23,38,49], unselected patients [19,21,24,26,29,31-37]. The intervention time of rectal NSAIDs was divided into three groups, before [19,24,26,29-37], during [20,23], or after [21,22,25,27,28] ERCP. In the studies with administration of rectal NSAIDs, only one study [19] involved three arms without placebo, the remaining studies included two arms, one type of rectal NSAID, and placebo. The total number of patients participating in the treatment was 5311, divided into the control group and the treatment group (2804 and 2507, respectively). In addition, the number of participants in stent therapy was 1,657, divided into a control group and a stent group of 827 and 830, respectively. Indomethacin was used in 11 studies [19,21-24,27,31-33,35,36], diclofenac in 8 studies [19,20,25,26,28-30,37], naproxen in 2 studies [19,34], and stents in 13 studies [38-50]. Sample sizes for the studies ranged from 19 to 665. The outcomes contain severe pancreatitis, moderate, incidence, and mild. Table 1 & 2 below specifically present all the features included in this network meta-analysis. Wang Yin and Zhang Qian mainly used the Cochrane collaboration tools to conduct migration risk assessment studies and conducted evaluation reviews for the quality of the methods they used. Each experiment was evaluated as a whole, including high risk, unknown risk and low risk. Among them, the reviewers shall deal with and solve the differences existing in the quality assessment. (Fu-Cheng Zhang). The summary of performance bias, reporting bias, attrition bias, selection bias and other biases identified for each individual RCT is shown in Figure 2a & 2b. All the results get from controlled trials showed that the migration risk was unclear or low.
Table 1: Characteristics of the included RCTs with rectal NSAIDs.
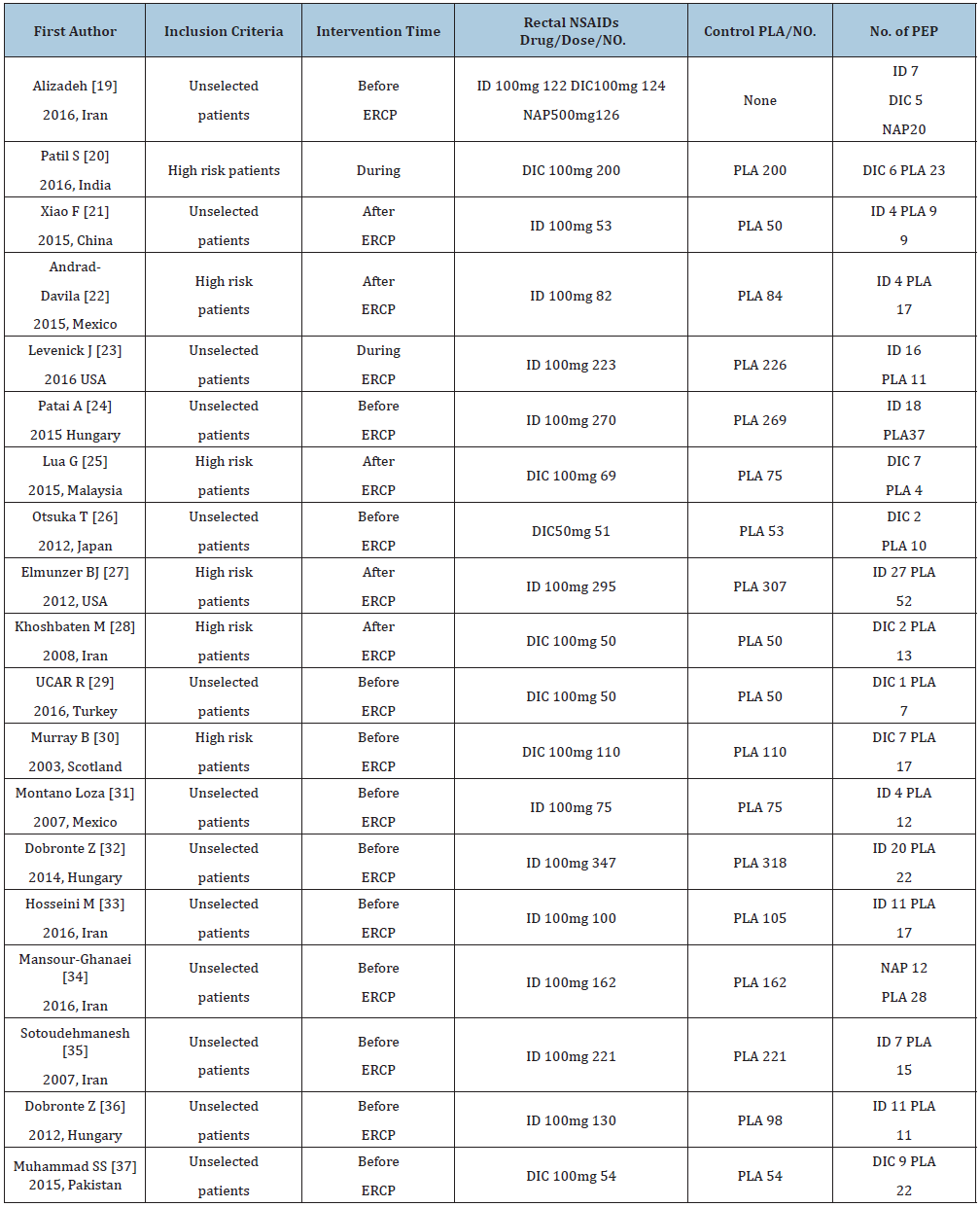
ID: Indomethacin; DIC: Diclofenac; NAP: Naproxen; PLA: Placebo; NO: Number; ERCP: Endoscopic retrograde cholangiopancreatography; PDS: Pancreatic duct stents; PEP: Post-Endoscopic retrograde cholangiopancreatography pancreatitis
Table 2: Characteristics of the included RCTs with pancreatic stents.
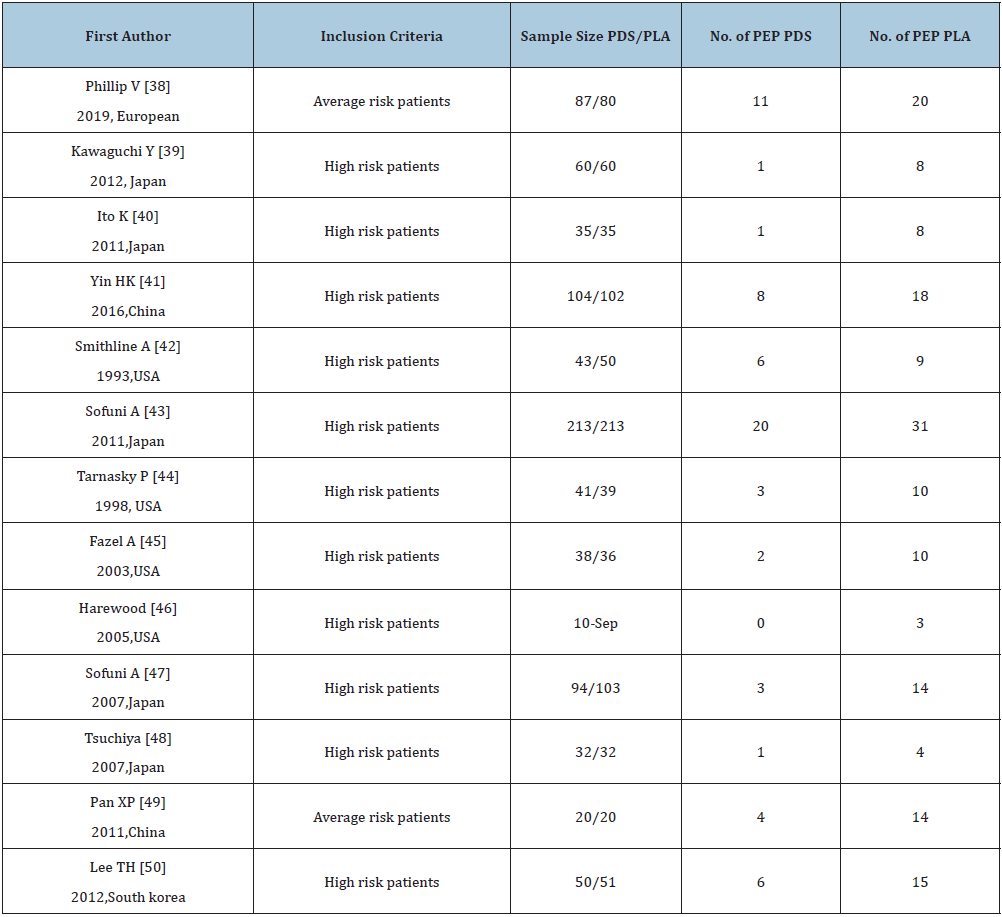
PDS: Pancreatic duct stents; PLA: Placebo; PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis.
Figure 2:
Standard meta-analysis
Combined with 18 comparative experiments, through the comparative analysis between placebo and rectal NSAIDS, among all the patients participating in the study, the participants in the control group and the intervention group were 2,542 and 2,507, respectively. At the same time, the random effect model was constructed for the analysis objects. It was known that the heterogeneity I2 between all the subjects participating in the study was 44.5%. A strong association was found between the treatment group and the incidence of PEP when compared with placebo (OR= 0.46, 95% CI 0.34 to 0.61, I2=44.5%). When we divided these trials into three subgroups based on the type of administration, intervention time, and the inclusion criteria, the type of administration indicated that the probability of occurrence of PEP showed a significant downward trend,DIC OR= 0.31 95% CI 0.16 to 0.59 I2=47.3%; ID OR= 0.56 95% CI 0.41 to 0.76 I2=33.8%; NAP OR= 0.38 95% CI 0.19 to 0.78. For intervention time, three subgroups - before, after and during ERCP - were analyzed, before ERCP OR= 0.44 95% CI 0.34 to 0.57 I2=7.2%; after ERCP OR= 0.41 95% CI 0.19 to 0.88 I2=61.1%; during ERCP OR= 1.51 95% CI 0.68 to 3.33. As for the standard of the inclusion criteria, two subgroups with high risk patients and unselected patients were analyzed, with high risk patients, OR= 0.37 95% CI 0.20 to 0.69 I2=57.8%; with unselected patients, OR= 0.51 95% CI 0.37 to 0.71 I2=36.1%. The various subgroup analyses carried out revealed a consistency between its results and the overall results, summarized in Figure 2c-2e. According to the stent placement vs placebo in the 13 trials, the total number of participants in the treatment trial was 827 in the intervention group and 830 in the control group. According to the random model, the heterogeneity between different study groups was 10.9%. Compared with placebo, PEPE has seen a significant decline in morbidity (OR= 0.34, 95% CI 0.24 to 0.49, I2=10.7%). When we divided these trials into two subgroups based on the inclusion criteria, patients (OR= 0.25, 95% CI 0.06 to 0.95, I2=63.2%) and high-risk patients (OR= 0.37, 95% CI 0.25 to 0.53, I2=5.0% (Figure 2f).
Figure 3:
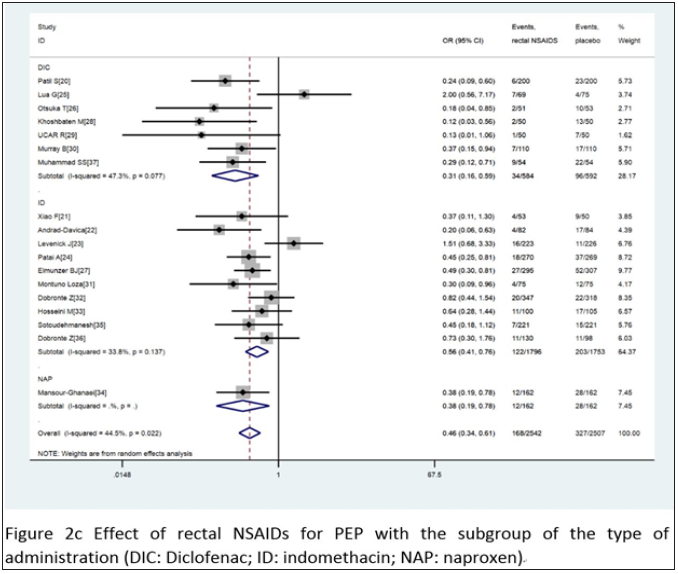
Figure 4:
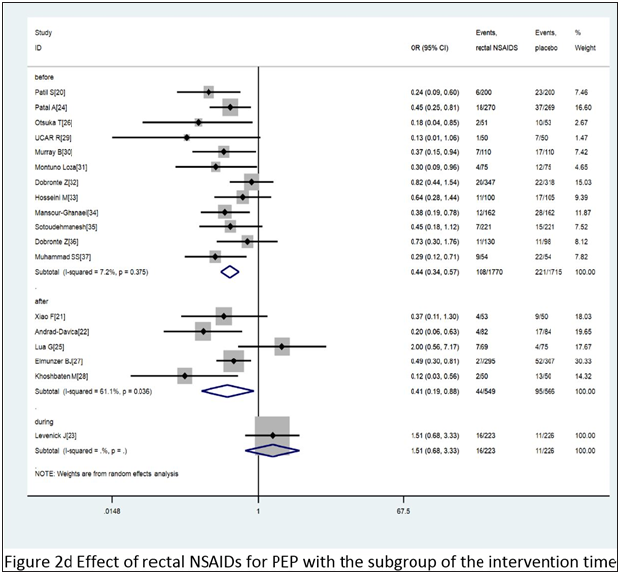
Figure 5:
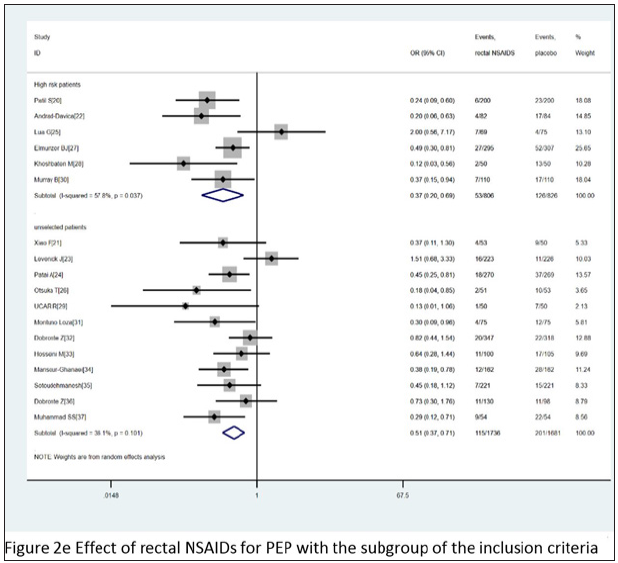
Figure 6:
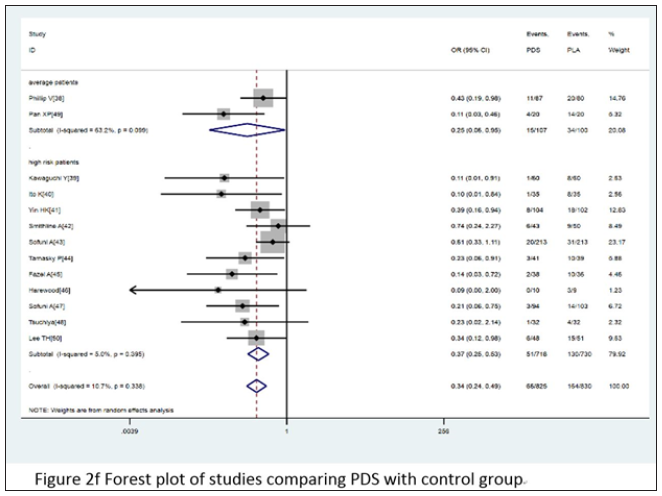
Figure 7:
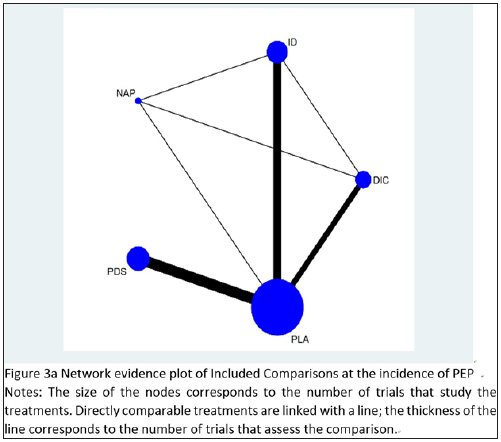
Network evidence plot
The network evidence plot of eligible comparisons for the incidence of PEP from all randomized controlled trials is shown in Figure 3a. The plot shows four closed loops, demonstrating ID-DIC-NAP, ID-NAP-PLA, ID-DIC-PLA, NAP-DIC-PLA. Because the rationale for the study design is that for any kind of network, stent therapy is not going to be compared to any other drug.
Contribution plot
The contribution plot for PEP is presented in detail in Figure 3b. In the mixed estimate, the result of DIC vs ID was derived from the direct comparison between ID vs PLA accounting for 35.6%, and with the mixed results of ID vs PLA, PLA vs PDS, coming from direct comparisons, taking the accounting to 79% and 100%. The indirect estimates, DIC vs PDS, ID vs PDS, NAP vs PDS, were from the direct comparison of PLA vs PDS, accounting for 41.5%, 47.1%, and 39.9%. The result of entire network was from the most weight among the direct comparisons, ID vs PLA accounting for 24% with 10 trials.
Figure 8:
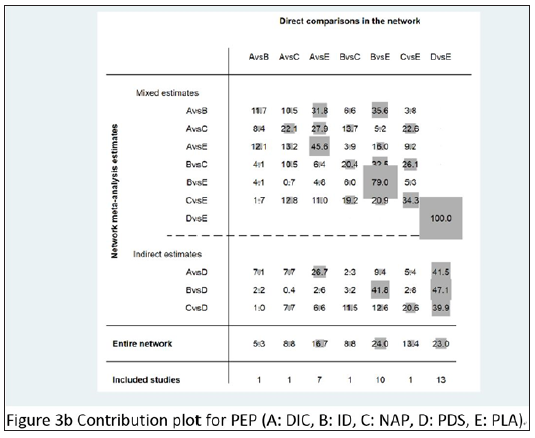
Figure 9:
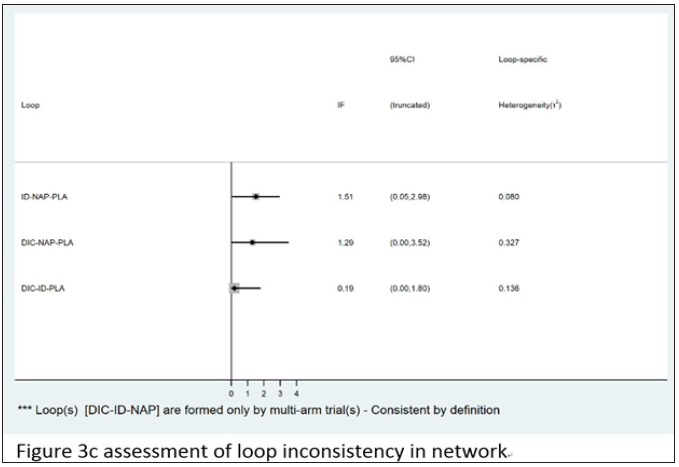
Loop consistency
Among the three closed loops, two loops DIC-NAP-PLA and DIC-ID-PLA indicates that there is no significant difference between indirect and direct comparisons. However, there was a significant difference between the simple comparison and the direct comparison of the id-nap -PLA cycle (P = 0.043 <0.05). The specific inconsistent assessment results are shown in Figure 3c.
Figure 10:
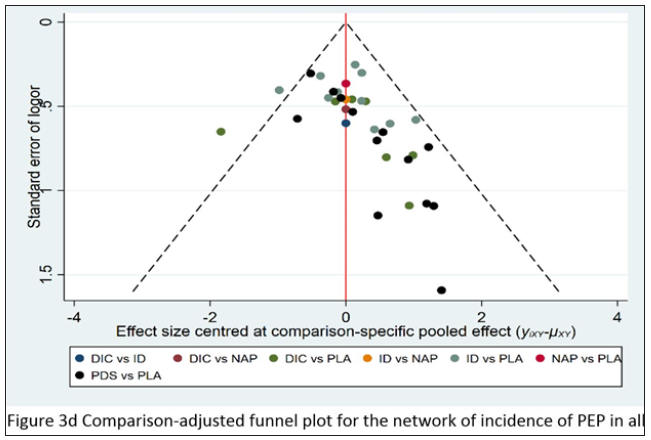
Comparison-adjusted plot
The comparison-adjusted funnel plot for the network of PEP incidence indicates that all pair comparisons are symmetrical, except for PDS vs placebo. Among the 13 papers, two trials [46,49] which were included has small participant number and may have an adverse effect on the publication bias as shown in Figure 3d.
Figure 11:
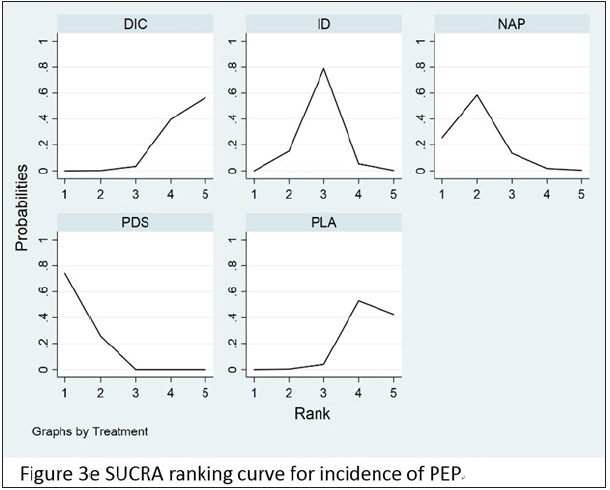
Core results for network
In order to compare and rank rectal NSAIDS and stent placement based on their ability to prevent PEP, the RCT data were taken as the target object and the network meta-analysis was carried out. According to the findings from the network meta-analysis using all trials, DIC (OR 0.30, 95% CI 0.18-0.49), ID (OR 0.52, 95% CI 0.37-0.74), NAP (OR 0.78, 95% CI 0.37-1.64), PDS (OR 0.32, 95% CI 0.21-0.48), DIC, ID, and PDS were all significantly associated with the reduction or prevention of PEP, whereas NAP was not. The results of network-analysis for preventing the incidence of PEP from all the trials is given in Table 3. Surface under the cumulative ranking curves (SUCRA curves) for preventing the incidence of PEP are presented in Figure 3e.
Table 3: Network estimated of treatments for incidence of PEP.
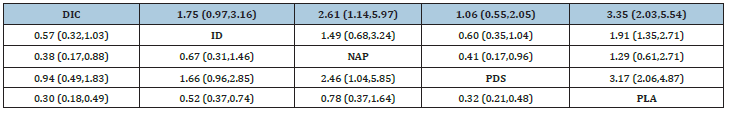
ID: Indomethacin; DIC: diclofenac; NAP: naproxen; PLA: placebo; PDS: pancreatic duct stents.
Figure 12:

Grade summary of evidence
The results of relevant studies on PEP prevention and the corresponding evidence are summarized below: indomethacin (high quality), naproxen (low quality), diclofenac (high quality), PDS (high quality).
Discussion
This network meta-analysis included all appropriate administrations often used by clinicians for preventing PEP, such as rectal NSAIDS or stent placement. The migration risk associated with all experiments is unclear or low. The results indicate that rectal NSAIDs and PDS significantly decrease the incidence of PEP when compared to placebo from the pairwise meta-analytic results. DIC and ID use demonstrate high quality evidence, there is a low level of quality for NAP related evidence with evidence from only one trial. Moderate heterogeneity can be seen with rectal diclofenac, in the trial from Lua G [25], it shows that there is no statistically significant difference between PLA and DIC. According to the results of the study on the use of NSAIDS in the rectum, the possibility of the occurrence of PEP can be greatly reduced, therefore, it is clear that the standard results are the same as the experimental results. For PDS, the incidence of PEP has been significantly reduced according to the results of some high-quality evidence.
In order to further improve the accuracy of the results we have obtained, so as to support clinical purposes, we now focus on further strengthening face-to-face studies and improving the credibility and quality of evidence. Our assessment overall found little difference between NSAIDs and PDS when all data were considered, except for NAP which had only one direct comparison, a network meta-analysis showed a lower level of quality. The remaining results were the same as the standard meta-analysis. At the same time, this ranking can indicate that the placement of stents is the best option among various ways to prevent PEP There was only one network meta-analysis comparing the rectal NSAIDs to stents.
We also make sure that the content is as comprehensive as possible when conducting literature search, including all published RCTs. Our research has several advantages as follows: first, we have expanded the study populations significantly. Second, this kind of network meta-analysis mainly includes the majority of which are high quality, however, the previous network meta-analysis included many retrospective experiments which may increase the heterogeneity and the credibility of results. Last, the present results indicate that placement of PDS was more effective for PEP prevention than the NSAIDs. There is clearly a significant difference between the results of this study and those obtained by conducting the meta-analysis described above. We first introduced the GRADE system to assess our results, which may be more helpful to clinicians, and compared the different types of rectal NSAIDs, and determined which type is the best choice for preventing PEP. Despite the above, we also have limitations in our experimental work that need to be addressed. Heterogeneities in some analyses including direct and mixed comparisons were moderate. According to the standard meta-analysis, we found that the heterogeneities were derived from the different types of NSAIDs. With the mixed comparisons, some loops were inconsistent, and through the funnel plot, we can tell that all PDS have the possibility of publication deviation, all the inconsistence and probable publication biases may be due to the number of direct comparisons among the rectal DIC, ID, NAP, and some small sample trials with PDS. Although the statistics suggest that placement of PEP stents were superior to rectal NSAIDs, this may not be ideal for daily clinical operations due to the complicated procedure, risk associated with the intervention, excess expense and inconvenience to the participants. Rectal NSAIDs may be the cheapest and most convenient choice, ESGE protocol recommends the use of rectal NASIDS to prevent PEP. However, the lack of direct comparisons between rectal NSAIDs and direct comparisons between rectal NSAIDs and PDS, suggest the need for further RCTs between stents and rectal NSAIDs to confirm our results. Taken together, stents and rectal NSAID can reduce the likelihood of PEP occurring. The available evidence from network meta-analysis suggests that stents are more effective in reducing the risk of PEP, because of the no significant contrast of rectal NSAID, there may be heterogeneity in our results, therefore, in the process of indirect comparison and direct comparison, high-quality RCT should be used as the basis to enhance the credibility of the results.
Author’s Contribution
Yin Wang: data collection, data extraction, data analysis and writing the article.
Qian Zhang: data collection, data extraction, data analysis and modifying the article.
Fu-Cheng Zhang: Technology and method guidance, handle differences.
References
- Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, et al. (2007) Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 102(8): 1781-1788.
- Masci E, Mariani A, Curioni S, Testoni PA (2003) Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: A meta-analysis. Endoscopy 35(10): 830-834.
- Talukdar R (2016) Complications of ERCP. Best Pract Res Clin Gastroenterol 30(5): 793-805.
- Kochar B, Akshintala VS, Afghani E, Elmunzer BJ, Kim KJ, et al. (2015) Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc 81(1): 143-149.
- Banks PA, Bollen TL, Dervebis C, Gooszen HG, Johnson CD, et al. (2013) Classification of acute pancreatitis- 2012: revision of Atlanta classification and definitions by international consensus. Gut 62(1): 102-111.
- Serrano JPR, de Moura DTH, Bernardo WM, Ribeiro IB, Franzini TP, et al. (2019) Nonsteroidal anti-inflammatory drugs versus placebo for post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review and meta-analysis. Endosc Int Open 7(4): E477-486.
- Lyu Y, Cheng Y, Wang B, Xu Y, Du W (2018) What is impact of nonsteroidal anti-inflammatory drugs in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: A meta-analysis of randomized controlled trials. BMC Gastroenterol 18(1): 106.
- Liu L, Li C, Huang Y, Jin H (2019) Nonsteroidal anti-inflammatory drugs for endoscopic retrograde cholangiopancreatography postoperative pancreatitis prevention: A systematic review and meta-analysis. J Gastrointest Surg 23(10): 1991-2001.
- Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, et al. (2015) Japanese guidelines for the management of acute pancreatitis: Japanese Guideline. J Hepatobiliary Pancreat Sci 22(6): 405-432.
- Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, et al. (2014) Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-updated June 2014. Endoscopy 46: 799-815.
- Mazaki T, Mado K, Masuda H, Shiono M (2014) Prophylactic pancreatic stent placement and post-ERCP pancreatitis: an updated meta-analysis. J Gastroenterol 49(2): 343-355.
- Wang Q, He XR, Tian JH, Yang KH (2013) Pancreatic Duct Stents at Pancreaticoduodenectomy: A Meta-Analysis. Dig Surg 30(4-6): 415-424.
- Shi QQ, Ning XY, Zhan LL, Tang GD, Lv XP (2014) Placement of prophylactic pancreatic stents to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients: A meta-analysis. World J Gastroenterol 20(22): 7040-7048.
- Akbar A, Abu Dayyeh BK, Baron TH, Wang Z, Altayar O, et al. (2013) Rectal nonsteroidal anti-inflammatory drugs are superior to pancreatic duct stents in preventing pancreatitis after endoscopic retrograde cholangiopancreatography: A network meta-analysis. Clin Gastroenterol Hepatol 11(7): 778-783.
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, et al. (2013) Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. CMAJ 185(4): E201-E211.
- Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomized trials. BMJ 343: d5928.
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11): 1539-58.
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, et al. (2008) What is ‘quality of evidence’ and why is it important to clinicians? BMJ 336(7651): 995-998.
- Mohammad Alizadeh AH, Abbasinazari M, Hatami B, Abdi S, Ahmadpour F, et al. (2017) Comparison of rectal indomethacin, diclofenac, and naproxen for the prevention of post endoscopic retrograde cholangiopancreatography pancreatitis. Eur J Gastroenterol Hepatol 29(3): 349-354.
- Patil S, Pandey V, Pandav N, Ingle M, Phadke A, Sawant P (2016) Role of Rectal Diclofenac Suppository for Prevention and Its Impact on Severity of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis in High-Risk Patients. Gastroenterology Res 9(2-3): 47-52.
- Fei X, Guang YW (2016) Effects of NSAIDS in preventing post-ERCP pancreatitis. Jilin medicine 5: 1-29.
- Andrade-Dávila VF, Chávez-Tostado M, Dávalos-Cobián C, García-Correa J, Montaño-Loza A, et al. (2015) Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC Gastroenterol 15: 85.
- Levenick JM, Gordon SR, Fadden LL, Levy LC, Rockacy MJ, et al. (2016) Rectal indomethacin does not prevent post-ERCP pancreatitis in consecutive patients. Gastroenterology 150(4): 911-917.
- Patai Á, Solymosi N, Patai ÁV (2015) Effect of rectal indomethacin for preventing post-ERCP pancreatitis depends on difficulties of cannulation: results from a randomized study with sequential biliary intubation. J Clin Gastroenterol 49(5): 429-437.
- Lua GW, Muthukaruppan R, Menon J (2015) Can rectal diclofenac prevent post endoscopic retrograde cholangiopancreatography pancreatitis? Dig Dis Sci 60(10): 3118-3123.
- Otsuka T, Kawazoe S, Nakashita S, Kamachi S, Oeda S, et al. (2012) Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial. J Gastroenterol 47(8): 912-917.
- Elmunzer BJ, Scheiman JM, Lehman GA, Kamachi S, Oeda S, et al. (2012) A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med 366(15): 1414-1422.
- Khoshbaten M, Khorram H, Madad L, Ehsani Ardakani MJ, Farzin H, et al. (2008) Role of diclofenac in reducing post-endoscopic retrograde cholangiopancreatography pancreatitis. J Gastroenterol Hepatol 23: e11-16.
- Uçar R, Biyik M, Uçar E, Polat İ, Çifçi S, et al. (2016) Rectal or intramuscular diclofenac reduces the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography. Turk J Med Sci 46(4): 1059-1063.
- Murray B, Carter R, Imrie C, Evans S, Suilleabhain C (2003) Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology 124(7): 1786-1791.
- Montaño LA, Rodríguez LX, García CJE, Dávalos CC, Cervantes GG, et al. (2007) Effect of the administration of rectal indomethacin on amylase serum levels after endoscopic retrograde cholangiopancreatography, and its impact on the development of secondary pancreatitis episodes. Rev Esp Enferm Dig 99(6): 330-336.
- Döbrönte Z, Szepes Z, Izbéki F, Gervain J, Lakatos L, et al. (2014) Is rectal indomethacin effective in preventing of post-endoscopic retrograde cholangiopancreatography pancreatitis? World J Gastroenterol 20(29): 10151-1057.
- Hosseini M, Shalchiantabrizi P, Yektaroudy K, Dadgarmoghaddam M, Salari M (2016) Prophylactic effect of rectal indomethacin administration, with and without intravenous hydration, on development of endoscopic retrograde cholangiopancreatography pancreatitis episodes: A Randomized Clinical Trial. Arch Iran Med 19(8): 538-543.
- Mansour-Ghanaei F, Joukar F, Taherzadeh Z, Sokhanvar H, Hasandokht T (2016) Suppository naproxen reduces incidence and severity of post-endoscopic retrograde cholangiopancreatography pancreatitis: Randomized controlled trial. World J Gastroenterol 22(21): 5114-5121.
- Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, et al. (2007) Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol 102(5): 978-983.
- Döbrönte Z, Toldy E, Márk L, Sarang K, Lakner L (2012) Effects of rectal indomethacin in the prevention of post-ERCP acute pancreatitis. Orv Hetil 153(25): 990-996.
- Muhammad SS, Jahangir SK, Muhammad UF, Shahbaz Z, Mubashir N, et al. (2016) Prophylactic rectal nsaids in the prevention of post-ERCP pancreatitis. J Postgrad Med Inst 30(2): 184-188.
- Phillip V, Pukitis A, Epstein A, Haf D, Schwab M, et al. (2019) Pancreatic stenting to prevent post-ERCP pancreatitis: A randomized multicenter trial. Endosc Int Open 7(7): E860-868.
- Kawaguchi Y, Ogawa M, Omata F, Ito H, Shimosegawa T, et al. (2012) Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol 18(14): 1635-1641.
- Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, et al. (2010) Can pancreatic duct stenting prevent post-ERCP pancreatitis in patients who undergo pancreatic duct guidewire placement for achieving selective biliary cannulation? A prospective randomized controlled trial. J Gastroenterol 45(11): 1183-1191.
- Yin HK, Wu HE, Li QX, Wang W, Ou WL, et al. (2016) Pancreatic stenting reduces post-ERCP pancreatitis and biliary sepsis in high-risk patients: a randomized, controlled study. Gastroenterol Res Pract: 9687052.
- Smithline A, Silverman W, Rogers D, Nisi R, Wiersema M, et al. (1993) Effect of prophylactic main pancreatic duct stenting on the incidence of biliary endoscopic sphincterotomy-induced pancreatitis in high-risk patients. Gastrointest Endosc. Gastrointest Endosc 39(5): 652-657.
- Sofuni A, Maguchi H, Mukai T, Kawakami H, Irisawa A, et al. (2011) Endoscopic pancreatic duct stents reduce the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Clin Gastroenterol Hepatol. Clin Gastroenterol Hepatol 9(10): 851-858.
- Tarnasky PR, Palesch YY, Cunningham JT, Mauldin PD, Cotton PB, et al. (1998) Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology 115(6): 1518-1524.
- Fazel A, Quadri A, Catalano MF, Meyerson SM, Geenen JE (2003) Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc 57(3): 291-294.
- Harewood GC, Pochron NL, Gostout CJ (2005) Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc 62(3): 367-370.
- Sofuni A, Maguchi H, Itoi T, Katanuma A, Hisai H, et al. (2005) Prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis by an endoscopic pancreatic spontaneous dislodgement stent. Clin Gastroenterol Hepatol 5(11): 1339-1346.
- Tsuchiya T, Itoi T, Sofuni A, Itokawa F, Kurihara T, et al. (2007) Temporary pancreatic stent to prevent post endoscopic retrograde cholangiopancreatography pancreatitis: a preliminary, single center, randomized controlled trial. J Hepatobiliary Pancreat Surg 14: 302-307.
- Pan XP, Dang T, Meng XM, Xue KC, Chang ZH, Zhang YP (2011) Clinical study on the prevention of post-ERCP pancreatitis by pancreatic duct stenting. Cell Biochem Biophys 61(3): 473-479.
- Lee TH, Moon JH, Choi HJ, Han SH, Cheon YK, et al. (2012) Prophylactic temporary 3F pancreatic duct stent to prevent post-ERCP pancreatitis in patients with a difficult biliary cannulation: A multicenter, prospective, randomized study. Gastrointest Endosc 76(3): 578-585.
© 2020 Yin Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






