- Submissions

Full Text
Gastroenterology Medicine & Research
The Mode of Action of NHE3 Inhibitors in Intestinal Na+ Absorption
Noriko Ishizuka1,2, Wendy Hempstock1,2 and Hisayoshi Hayashi1*
1Laboratory of Physiology, School of Food and Nutritional Sciences, University of Shizuoka, Japan
2Both authors contributed equally to the work
*Corresponding author:Hisayoshi Hayashi, Laboratory of Physiology, School of Food and Nutritional Sciences, University of Shizuoka, Japan
Submission: November 18, 2019;Published: November 22, 2019

ISSN 2637-7632Volume4 Issue1
Abstract
Transport activity of Na+/H+ exchanger is sensitive to intracellular pH (pHi). At resting pHi, a large fraction of transporters resides in an inactive state. When the H+ concentration of the cytosol rises, the transporters are converted into an active state. We have previously shown that intestinal Na+/H+ exchanger 3 isoform (NHE3) is slowly activated over the course of minutes, implying involvement of a conformational change of NHE3. Recently, some NHE3 specific inhibitors have been developed to treat sodium-fluid imbalance diseases. However, the action of these inhibitors on NHE3 is not fully elucidated. To gain insight into the inhibition mechanism of NHE3 inhibitors, we used exogenous NHE3 expressing cells and mouse intestine. Our results suggested that tenapanor, which has a symmetrical structure with two proposed binding sites, may irreversibly bind to NHE3 and recognize the different NHE3 transport modes.
Introduction
Na+/H+ exchangers (NHEs) are electroneutral transporters that mediate a one for one exchange of extracellular sodium and intracellular protons. NHE isoform 3(NHE3) is expressed predominantly in the apical membrane of intestinal epithelia, where it plays a pivotal role in NaCl absorption and acid-base homeostasis. NHE3 is thought to work in different transport modes, where NHE3 is coupled to a nutrient transporter, such as SGLT1, PepT1, etc. Inhibition of NHE3 is implicated in a number of diarrheal diseases, however, recently NHE3 inhibitors have been getting attention for their use in treatment of imbalances caused by excessive sodium intake or decreased intestinal excretion of sodium (hyperabsorption of sodium). Sodium-fluid imbalances in the intestine are implicated in diseases such as hypertension, constipation-predominant irritable bowel syndrome, hyperphosphatemia and in chronic kidney disease (CKD) [1].
{3-[2-(3-guanidino-2-methyle-3-oxo-propenyl)-5-methyl-phenyl]-N-isopropylidene-2- methyl-acrylamide dihydrochloride} (S3226) was first identified as a selective NHE3 inhibitor in studies with cells and porcine renal brush-border membrane vesicles [2]. Intravenously administered S3226 was found to improve glomerular filtration rate and kidney function in Ischemia-induced acute renal failure rats [3]. Two other NHE3 specific inhibitors have been developed to treat hypertension without being absorbed by the intestine: SAR218034 (SAR) and tenapanor hydrochloride (hereafter, tenapanor). SAR administration in spontaneously hypertensive rats led to increased fecal water content as well as a reduction in systolic blood pressure [4]. In addition to treating hypertension, tenapanor and SAR have the potential to prevent CKD progression by preventing hypertension and fluid overload [5]. In humans and rats, tenapanor reduced urine sodium levels while sodium levels in the stool were increased [6]. Tenapanor has been found to be well tolerated in healthy human volunteers without affecting plasma electrolytes, although it was found to increase stool softness [6]. Tenapanor also protects against vascular calcification in a rat model of CKD by inhibiting phosphate absorption [7]. Since tenapanor seems to be well tolerated in studies with healthy human volunteers, it is a good candidate for patients with CKD or constipation-predominant irritable bowel syndrome [8].
The NHE3 inhibitors SAR and tenapanor appear to work by reversibly binding to NHE3 and inhibiting sodium uptake, thus reducing sodium in the urine and increasing sodium in the stool [5], however to our knowledge, there are no studies supporting this mechanism of action. The mechanism behind decreased phosphate absorption is due to tightening of the tight junctions and reducing paracellular phosphate permeability via NHE3 inhibition and decreasing the expression of the phosphate transporter NaPi2b [9]. However, to understand the action of NHE3 more clearly, a better understanding of how NHE3 inhibitors such as tenapanor and S3226 work is required.
Activation of NHE3 transport involves a change in pHi. At resting pHi, a large fraction of transporters resides in an inactive state. When the H+ concentration of the cytosol rises, the transporters are converted into an active state. One explanation for this regulation of transport activity is the existence of not only a H+ transport site, but also an intracellular H+ modifier site that allosterically regulates transport activity [10]. Another proposed explanation is that the transporter exists as a symmetrical dimer model (Monod-Wyman- Changeux model). During intracellular acidification, the low-affinity form for intracellular H+ is converted into a form harboring a higher affinity for intracellular H+ which does not require an additional intracellular H+ site [11].
We have previously shown that NHE3 is slowly activated over the course of minutes [12]. Activation of NHE3 was not due to an increased number of transporters at the cell surface, nor was it due to changing the phosphorylation state. This slow activation mode implies the involvement of a conformational change of NHE3 such as dimerization. Tenapanor can theoretically bind NHE3 dimers, however the mechanism of action is still unclear so insight to the inhibitor mechanism of NHE3 may also help to understand the activation mechanism. Therefore, to gain insight into the inhibition mechanism of NHE3 inhibitors, we used exogenous NHE3 expressing cells and mouse intestine.
Material and Methods
Measurement of NHE3 activity in NHE3 expressing cells
PS120 cells, which lack endogenous Na+/H+ exchange activity [13], were used and NHE3 stable transfect ants were made. NHE3 expressing PS120 cells were grown on glass coverslips and stained with pH-sensitive fluorescent dye BCECF. The activity of NHE3 was determined as the rate of Na+-induced pHi recovery after acid loading.
Measurement of NHE3 activity in the mouse intestine
The middle third of the small intestine was used for peptide experiments and the middle section of the colon was used for measurement of 22Na+ flux. Each isolated segment was opened, and the muscle layer was stripped off and then mounted in an Ussing chamber. Measurement of electrical parameters under short-circuit conditions were performed as previously [14].
Results
Effect of tenapanor on NHE3 activity at different acidified durations
In NHE3 expressing cells, tenapanor inhibited NHE3 activity dose-dependently with IC50 values of 1.26±0.40nM (n=4) and 3.02±0.53nM (n=3) for 1min and 10min acidification, respectively. This result implies that the chemical structure of tenapanor, which has a symmetrical structure with two proposed binding sites, is not involved in the slow activation mechanism of NHE3.
S3226 completely inhibits transepithelial 22Na+ flux but tenapanor does not
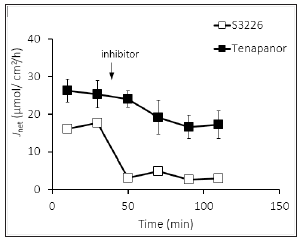
Bulk transport of NaCl absorption in the colonic epithelium is mediated by electroneutral absorption by luminal Na+/H+ exchanger NHE3 and Cl-/HCO3- exchanger DRA. The unidirectional transmural 22Na+ fluxes of mucosal-to-serosal (JMS) and serosal-tomucosal (JSM) were measured in adjacent tissues. Net22Na+ flux (JNet) was calculated by subtracting JSM from JMS. The addition of S3226 to the luminal side resulted in complete inhibition of JNet (Figure 1). However, upon 1μM tenapanor application, which is 100 times higher concentration than the IC50 value, partial inhibition of JNet was observed.
Figure 1:Inhibition of Net22Na+ flux by S3226 and tenapanor.
Tenapanor and S3226 dose-dependently inhibit increment of peptide-induced short circuit current
H+-coupled peptide transporter (PepT1; slc15a1) exists in the brush border membrane in the small intestine. We have previously shown that Glycyl-sarcosine (non-hydrolysable dipeptide; Gly-Sar) induces Isc increments that were tightly coupled to luminal Na+/H+ exchange activity [14]. After Gly-Sar -induced short circuit current (Isc) was attained at a plateau level, the addition of tenapanor or S3226 to the mucosal side resulted in inhibition of Isc by a dosedependent manner with an IC50 value of 6.3±3.4nM (n=3) and 5.9±1.0μM (n=3) for tenapanor and S3226, respectively (figure not shown). Unlike inhibition of colonic Na+ absorption, the magnitude of inhibition was not different between each inhibitor (about 70% of Gly-Sar-induced Isc). These results suggest that tenapanor may recognize the different NHE3 transport modes.
Pretreatment with tenapanor dose-dependently inhibits NHE3 activity
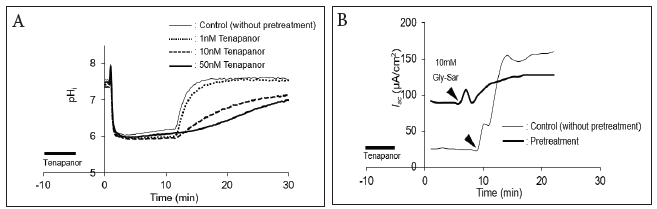
NHE3-expressing cells were treated with various concentrations of tenapanor for 5min and washed with Ringer’s solution. NHE3 activity was measured as the rate of pHi recovery (Figure 2A). Tenapanor dose-dependently inhibited NHE3 activity with an IC50 value of 5.8nM. These results suggest that tenapanor may irreversibly bind to NHE3. To assess this phenomenon in native tissue, we measured peptide induced Isc after pretreatment with tenapanor (Figure 2B). Before measurement of Isc, 100nM tenapanor was added to the luminal side for 5min and then washed with Ringer’s solution. In the absence of tenapanor, Gly-Sar-induced Isc was strongly inhibited.
Figure 2:Pretreatment with tenapanor dose-dependently inhibits NHE3 activity. A. The cells were treated with various concentrations of tenapanor for 5min (indicated by solid line) and washed with Ringer’s solution. B. Before measurement of Isc, 100nM tenapanor was added to the luminal side for 5min (indicated by solid line) and then washed with Ringer’s solution.
Concluding remarks
Our results suggested that tenapanor, may irreversibly bind to NHE3 and recognize the different NHE3 transport modes. However, elucidation of the inhibition mechanisms of tenapanor needs further studies.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 17K12904 (to N.I.). Wendy Hempstock is a recipient of Otsuka Toshimi Scholarship Foundation scholarship from 2018 to 2020.
References
- Gurney MA, Laubitz D, Ghishan FK, Kiela PR (2017) Pathophysiology of intestinal Na+/H+ Cell Mol Gastroenterol Hepatol 3(1): 27-40.
- Schwark JR, Jansen HW, Lang HJ, Krick W, Burckhardt G, et al. (1998) S3226, a novel inhibitor of Na+/H+ exchanger subtype 3 in various cell types. Pflugers Arch 436(5): 797-800.
- Hropot M, Juretschke HP, Langer KH, Schwark JR (2001) S3226, a novel NHE3 inhibitor, attenuates Ischemia-induced acute renal failure in rats. Kidney Int 60(6): 2283-2289.
- Linz D, Wirth K, Linz W, Heuer HOO, Frick W, et al. (2012) Antihypertensive and laxative effects by pharmacological inhibition of sodium-proton-exchanger subtype 3-Mediated sodium absorption in the gut. Hypertension 60(6): 1560-1567.
- Spencer AG, Greasley PJ (2015) Pharmacologic inhibition of intestinal sodium uptake: A gut centric approach to sodium management. Curr Opin Nephrol Hypertens 24(5): 410-416.
- Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, et al. (2014) Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 6(227): 227ra36.
- Labonté ED, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, et al. (2015) Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 26(5): 1138-1149.
- Rosenbaum DP, Yan A, Jacobs JW (2018) Pharmacodynamics, safety, and tolerability of the NHE3 inhibitor Tenapanor: Two trials in healthy volunteers. Clin Drug Investig 38(4): 341-351.
- King AJ, Siegel M, He Y, Nie B, Wang J, et al. (2018) Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med 10(456): 1-18.
- Otsu K, Kinsella JL, Koh E, Froehlich JP (1992) Proton dependence of the partial reactions of the sodium-proton exchanger in renal brush border membranes. J Biol Chem 267(12): 8089-8096.
- Lacroix J, Poët M, Maehrel C, Counillon L (2004) A mechanism for the activation of the Na/H exchanger NHE-1 by cytoplasmic acidification and mitogens. EMBO Rep 5(1): 91-96.
- Hayashi H, Szaszi K, Coady-Osberg N, Orlowski J, Kinsella JL, Grinstein S (2002) A slow pH-dependent conformational transition underlies a novel mode of activation of the epithelial Na+/H+ exchanger-3 isoform. J Biol Chem 277(13): 11090-11096.
- Pouyssegur J, Sardet C, Franchi A (1984) A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci 81(15): 4833-4837.
- Ishizuka N, Nakayama M, Watanabe M, Tajima H, Yamauchi Y, et al. (2018) Luminal Na+ homeostasis has an important role in intestinal peptide absorption in vivo. Am J Physiol Gastrointest Liver Physiol 315(5): G799-G809.
© 2019 Hisayoshi Hayashi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






