- Submissions

Full Text
Gastroenterology Medicine & Research
Irritable Bowel Syndrome and Intestinal Microbiote Transplantation
Álvaro Zamudio-Tiburcio1*, Héctor Bermúdez-Ruiz2 and Pedro Antonio Reyes- López3
1 Department of Gastroenterology, México
2 Department of Endoscopy, México
3 Department of Cardiology, México
*Corresponding author:Álvaro Zamudio Tiburcio, Department of Gastroenterology, México
Submission: April 17, 2019;Published: April 30, 2019

ISSN 2637-7632Volume3 Issue1
Summary
We present 14 cases of patients who had IBS, half women and half men, who underwent intestinal microbiota transplantation. Gastrointestinal symptoms and signs are discussed. Abdominal pain and increased abdominal diameter appear in all cases, as well as their results. The concurrent diagnoses are indicated, with the appearance of Anxiety in all reported cases. Which decreased in all of them, once the TMI was instilled. We discuss what happened in relevant cases such as Microbiota Disease, Amyotrophic Lateral Sclerosis and Fibromyalgia, where the results were encouraging. Psoriasis on plate decreased, although it did not yield. Finally, comments are made, where emphasis is given to the Microbiota Disease, of which special considerations are made.
Abbreviations: IBS: Irritable Bowel Syndrome; FMT: Fecal Microbiota Transplantation; IMT: Intestinal Microbiota Transplantation; IM: Intestinal Microbiota
Presentation of Cases
We studied 14 patients, seven female and seven male. With ages ranging from 35 to 83 years. Nine of them presented IBS-D; four SII-M and only one SII-E.
Gastrointestinal symptoms and signs were
1. Abdominal pain 14 cases
2. Abdominal diameter increased 14 cases
3. Changes in bowel habits 12 cases
4. Diarrhea 9 cases; from 3 to 5 evacuations a day
5. Diarrhea and constipation 4 cases
. Constipation 1 case
7. Bloating 1 case
What happened to these Hallagos?
In cases of abdominal pain, it decreased from 25% to 75%. Not all cases of increased abdominal diameter gave way. 1 patient increased the abdominal diameter 2 centimeters. The decrease in abdominal diameter was less than 2 centimeters to 14. In the 12 cases of changes in bowel habits, in one, they disappeared and, in the rest,, they decreased from 25% to 75%. Diarrhea, except for 1 patient, was reduced. In the 4 cases of IBS-M, there was reduction of both diarrhea and constipation (Tables 1 & 2). In addition, we detected the following unique diagnoses: Alcoholism, Allergy to cold, Complete arrhythmia due to atrial fibrillation, Spastic colon, Microbiota disease, Amyotrophic lateral sclerosis, Fibromyalgia, Medicated gastropathy, Hearing loss, Peripheral vascular insufficiency, Grade II Listesis (L- 2), uterine myomatosis, facial neuralgia, plaque psoriasis, low digestive tract bleeding and vulvar vaginitis. The Disease of the Microbiota and the Fibromyalgia yielded totally to the 5 and 14 days, respectively. In amyotrophic lateral sclerosis, after 1 year of follow-up, the patient only presents minimal discomfort in the knees.
Table 1:
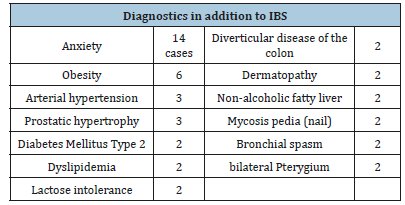
Table 2:
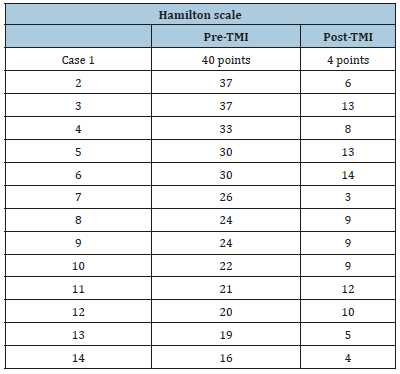
In Plate Psoriasis we have seen improvement, although no remission. Among the post-endoscopic findings, we find:
1. Rectal adenomas, spastic colon, rectal vascular ectasia, Barret’s disease, diverticular colon disease, drug gastropathy, hiatal hernia and gastric polyps (excised). Of these conditions only spastic colon improved 40%.
2. Post-MI complications occurred in 2 patients. In one of them there was diarrhea with 8 bowel movements and abdominal pain. They gave in a week with the administration of probiotics and antispasmodics.
3. The other case had diarrheal evacuations in number of 2; They lasted 2 days and, they gave in spontaneously.
Comments
The disease in which the Fecal Microbiota Transplant (TMF) has been most studied is Clostridium difficile affectation, followed by Irritable Bowel Syndrome (IBS) [1-6]. Given that, in both pathologies, the results are usually good, we consider it necessary to widely recommend this procedure. Something that happens very often and, therefore, has attracted our attention is that, in cases of IBS, anxiety is usually present, in its different percentages. We evaluated it based on the Hamilton survey, and we observed that in all cases there is reduction of anxiety, which indirectly produces improvements in other devices and systems. Several reports show that IMT has a favorable impact on the symptoms of various systems, especially in psychiatric disorders, anxiety type, skin disorders, various digestive disorders, degenerative disorders and painful joint processes, as well as several neuropathies-psychiatric disorders [7-9].
Since the pathogenesis in IBS is multifactorial, it is believed to be due to a complex interaction between the “bowel and brain axis”, the immune system and the Intestinal Microbiota (MI) [10- 12]. When the MI is altered, it produces the so-called dysbiosis, which is nothing more than its intrinsic changes and this generates modifications, both in gastrointestinal motility, and in the sensitivity of the same System that leads to an increase in this senility, which translates to MI intervenes in the pathophysiology of IBS [13,14].
Something very studied are the changes that occur when the SII is the result of infection. The patient experiences clinical symptoms of acute gastroenteritis [15], a fact that increases the percentage of the condition which translates into an increase of 6 to 7 times in the development of the disease. It is proposed that the pathogens that cause acute gastroenteritis release cytohelicoid toxin and, through molecular mimicry and autoantibodies against vinculin (a native cytoskeletal protein), can cause similar symptoms and alteration of intestinal motility, as observed in the SII and in the eradication of bacterial growth, resulting in some normalization of motility [16]. There are patients with early IBS with constipation who have a larger population of sulfate-reducing bacteria compared to healthy controls [17]. The Microbiota Disease is a significant disease that occurs in millions of people and that, fortunately, can be improved by the substitution of diseased or healthy microbiota. This disease, caused by imbalances in the intestinal microbiome, translates different conditions, among which are Anxiety, Intestinal Inflammatory Disease, Allergies, Irritable Colon Syndrome, Celiac Disease, Metabolic Syndrome, Asthma, some Cardiovascular Diseases and the obesity [18-22]. Some authors point to the Disease of the Microbiota as Dysbiosis (Dysbacteriosis) and, we consider that the dysbiosis produces the Disease of the Microbiota and consequently, this last one, can produce multiple manifestations, in diverse apparatuses or systems, as they are the digestive phenomenon, dermatological, psychiatric, inflammatory, immunological and other disorders [23-25].
Conclusion
The priority in the Intestinal Microbiota Transplant (TMI) is the selection of an excellent donor [26], in whom all the necessary studies are carried out, in order to avoid transmitting any condition. We currently prefer adults under 25 years old. In the near future, the ideal donor will be children. We prefer to use the jejunum or colon in the TMI. If there is a high digestive pathology, we use the jejunum. If there is low digestive pathology, we use the colon and if there is digestive pathology both high and low, we use both the jejunum and the colon.
The amount of microbiota to be transplanted must be at least 500 milliliters, of which it is generally “250 grams of microbiota and the rest is diluent”. In our experience, we have noticed that when the jejunal and colonic tracts are used, transplanting more than 800 milliliters of microbiota, the results are much better.
IMR is a harmless procedure and complications, if present, are usually reversible. We do not expect total cures, although the answer is magnificent, the corrections are usually between 40 and 70% improvement. The literature reports that in some patients two or more transplants are required to improve their clinical status. [27-29]. In our patients with IBS there has been no need, to date, in which we have 3 years of follow-up. The literature also reports that there is a reversal of TMI generally per year. We have not noticed this circumstance yet [30-32]. The results of the improvement shown by the patients have fluctuated between 2 days and 2 weeks from the date on which the transplant was performed. Our casuistry is not very high, however, the benefits of the TMI and the rapid and good response in IBS patients are striking.
References
- Jan G, Ferenc S, Magdalena K, Paprocka-Borowicz M, Piotr D, et al. (2016) Secondary aortoenteric fistula after abdominal aortic graft implementation in our own material. Adv Clin Exp Med 25(6): 1265- 1271.
- Sojun H, Koichi A, Masaya S, Toshihiro K, Hitoshi S (2016) Successful resolution of a hemorrhagic pancreatic pseudocyst ruptured into the stomach complicating obstructive pancreatitis due to pancreatic cancer: A case report. Hoshimoto World Journal of Surgical Oncology 14: 46.
- Vernadakis S, Christodoulou E, Treckmann J, Saner F, Paul A, et al. (2009) Pseudoaneurysmal rupture of the common hepatic artery into the biliodigestive anastomosis. A Rare Cause of Gastrointestinal Bleeding. JOP 10(4): 441-444.
- Chin CC, Yeh CY, Kuo YH, Wang JY (2008) Massive lower gastrointestinal bleeding from an external iliac artery fistula in a patient with bladder cancer. Chang Gung Med J 31(6): 612-615.
- Nirmit D, Sagar P, Chinyere N, Lok S, Carl T, et al. (2013) Arteriojejunal fistula presenting with recurrent obscure gi hemorrhage in a patient with a failed pancreas allograft. Case Rep Transplant 2013: 4.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, et al. (2010) A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: A review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 4(3): 510-517.
- Muñoz-Villafranca C, García-Kamirruaga Í, Góme-García P, Atín-del- Campo V, Bárcena-Robredo V, et al. (2015) Pseudoaneurysm of the cystic artery: An uncommon cause of upper gastrointestinal bleeding in a case of xanthogranulomatous cholecystitis. Rev Esp Enferm Dig 107(6): 375- 376.
- Lazaris AM, Tsapralis D, Patapis P, Mproutzos E, Tzathas H, et al. (2009) Aortoiliac endograft-enteric fistula due to an ingested toothpick. J Vasc Surg 50(3): 640-643.
- Um SJ, Park BH, Son C (2009) An Aortoesophageal fistula in patient with lung cancer after chemo-irradiation and subsequent esophageal stent implantation. J Thorac Oncol 4(2): 263-265.
- Karthaus EG, Post IC, Akkersdijk GJ (2016) Spontaneous aortoenteric fistula involving the sigmoid: A case report and review of literature. Int J Surg Case Rep 19: 97-99.
- Pérez-Legaz J, Marín-Hargreaves G, Ramírez M, Moya P, Arroyo A (2013) Renal-appendicular fistula of the renal graft in a transplanted patient: an uncommon form of lower gastrointestinal hemorrhage. Cir Esp 91(6): 397-399.
- Zhou JC, Xu QP, Shen LG, Pan KH, Mou YP (2009) Aortoduodenal fistula following aortic reconstruction of a pseudoaneurysm caused by stab wound 12 years ago. J Zhejiang Univ Sci B 10(5): 400-403.
- Marek T, Hendryk V, Christian W, Reimer A (2014) Aortoenteric Fistula as a Complication of Open Reconstruction and Endovascular Repair of Abdominal Aorta. Radiol Res Pract 2014: 6.
- Moulakakis KG, Kakisis J, Dalainas I, Liapis CD, Smyrniotis V (2015) Endovascular Management of Secondary Aortoduodenal Fistula: The Importance of Gut Restoration. Int J Angiol 24(1): 55-58.
- Bas A, Simsek O, Kandemirli SG, Rafiee B, Gulsen F, et al. (2015) Evolution of Computed Tomography Findings in Secondary Aortoenteric Fistula. Iran J Radiol 12(2): e22759.
- George E, Philippakisa, Marios M (2013) Surgical treatment of primary aortojejunal fistula. Int J Surg 4 (5): 477-479.
- Costea R, Vasiliu EC, Zărnescu NO, Neagu S (2015) Primary Aortoenteric Fistula: Case Report. Chirurgia (Bucur) 110(1): 78-80.
- Shulik O, Marling K, Butler J (2013) Primary aorto-enteric fistula-A unique complication of poorly differentiated large B-cell lymphoma. Am J Case Rep 14: 194-197.
- Guner A, Mentese U, Kece C, Kucuktulu U (2013) A rare and forgotten diagnosis of gastrointestinal bleeding: Primary aortoduodenal fistula. BMJ Case Rep 2013.
- Ahmed S, Patel C, Schmitt C, Parajuli D (2016) A Deadly Connection: Aortoenteric Fistula as a Cause of Acute Upper Gastrointestinal Bleeding. ACG Case Reports J 3(3): 152-153.
- Ho S, Liu B, Loya R, Koury I (2016) Primary aortoenteric fistula: A rare case of a massive gastrointestinal bleed. Cureus 8(9): e766.
- Malik MU, Ucbilek E, Sherwal AS (2015) Critical gastrointestinal bleed due to secondary aortoenteric fistula. J Community Hosp Intern Med Perspect 5(6): 29677.
- Varetto G, Gibello L, Trevisan A, Castagno C, Garneri P, et al. (2015) Primary Aortoenteric Fistula of a Saccular Aneurysm. Korean Circ J 45(4): 337-339.
- Fernández de Sevilla E, Echeverri JA, Boqué M, Valverde S, Ortega N, et al. (2015) Life-threating upper gastrointestinal bleeding due to a primary aorto-jejunal fistula. Int J Surg Case Rep 8C: 25-28.
© 2019 Álvaro Zamudio-Tiburcio. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






