- Submissions

Full Text
Gastroenterology Medicine & Research
A Rare Case of Helicobacter Pylori-Negative Gastric- MALT Lymphoma, Disseminated to the Small bowel, Colon, and Lung
Micaella Kantor1*, Monica Multani1, Nimal Patel 1, Javier Sobrado2 and Cristina Marin2
1 Department of Internal Medicine, USA
2 Department of Gastroenterology, USA
*Corresponding author: Micaella Kantor, Department of Internal Medicine, Florida, USA
Submission: September 26, 2018;Published: November 27, 2018

ISSN 2637-7632Volume2 Issue3
Abstract
Extranodal marginal zone lymphomas of mucosa-associated lymphoid tissue (EMZL-MALT) have been linked to chronic immune stimulation due to infection or autoimmune stimuli. Helicobacter Pylori induced gastric MALT lymphoma is the most extensively documented and well understood. However, in those where H. pylori is not detected, data is less established. Its treatment and prognosis are based on histological evaluation, immunophenotyping, and the use of fluorescence in situ hybridization (FISH), or polymerase chain reaction (PCR) to identify chromosomal translocation and/or unbalanced aberrations. The most common chromosomal translocation with pathogenic significance is t (11;18) (q21:q21). Those patients presenting with this translocation tend to have more advanced disseminated disease and are likely resistant to conventional antibiotic therapy and certain chemotherapeutic agents. Synchronous MALT lymphoma and overt distant dissemination has rarely been reported. We report a case of a 71-year-old female with biopsy confirmed H. pylori negative-gastric MALT lymphoma with disseminated disease involving the stomach, small intestine, colon, and lung. FISH analysis was positive for BIRC-3-MALT1 (11;18) fusion protein. Finally, we issue a call-to-action for much needed further data to establish clear treatment options based on clinical and diagnostic criteria, for extra-gastric, disseminated, and/or H. pylori negative MALT lymphomas.
Keywords: Mucosa-associated lymphoid tissue lymphoma; Metastasis; Helicobacter Pylori; t(11;18) (q21:q21); Bronchus-associated lymphoid tissue lymphoma
Introduction
Extranodal marginal zone lymphomas of mucosa-associated lymphoid tissue (EMZL-MALT) is a relatively rare subtype of lowgrade non-Hodgkin lymphomas [1,2]. It accounts for approximately 3-7% of all B-cell lymphomas [1,3]. They originate from memory B lymphocytes normally present in the “marginal zone” of the secondary lymphoid follicle found in mucosa-associated lymphoid tissue of various organs [4,5]. This clonal B-cell neoplasm has the ability to not only recur locally, but also to transform to a more aggressive diffuse large B-cell lymphoma with potential for systemic spread [2,5,6].
The stomach is the most commonly affected organ and Helicobacter Pylori (H. pylori) is the culprit in up to 90% of the cases [2,7-9]. In the remaining 10% of gastric MALT lymphomas, no H. pylori is detected which renders resistance to antibiotic therapy [5,9,10]. Though the pathogenesis of H. pylori- negative gastric MALT lymphoma is poorly understood, PCR analysis has shown that certain chromosomal abnormalities are often present in high frequencies. For instance, t(11:18) (q21;q21) occurs in up to 40% H. pylori-negative MALT lymphomas which may confer an independent growth advantage [5,9,11]. These neoplasms not only tend to be more aggressive, but also present at advanced stages with regional lymph nodes, pulmonary, and/or bone marrow [12,13]. We report a rare case of a 71-year-old female with biopsy confirmed H. pylori negative- gastric MALT lymphoma with disseminated disease involving the stomach, small intestine, colon, and lung.
Case Presentation
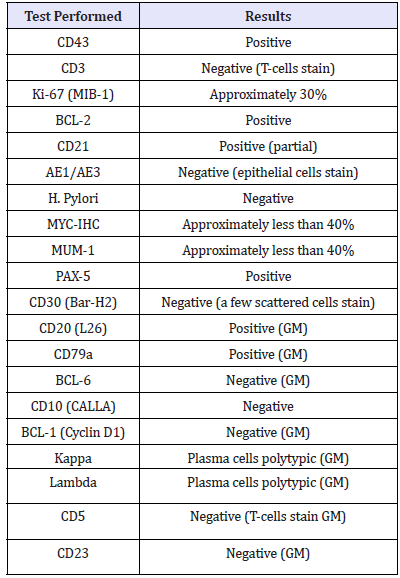
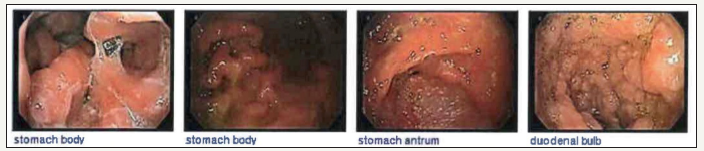
A 71-year-old female, with history of chronic, watery diarrhea and a ten-pound weight loss, was referred from her primary care physician for a screening colonoscopy. She had a recent CT scan of the chest with intravenous contrast showing multiple bilateral lung nodules concerning for metastatic disease. A PET scan showed increased uptake in the largest nodule of the right upper lobe of the lung, within the central mesenteric left perisigmoid lymph nodes, and in the left lymph node around the left common iliac artery. Upper endoscopy showed a normal esophagus with diffuse continuous congestion, erosions, erythema, friability, granularity, ulcerations and nodularity of the gastric mucosa, compatible with an infiltrative process. No active bleeding was noted. Biopsies were taken of the gastric antrum, body, and fundus. Pathology revealed atypical lymphoid proliferation with ulceration, active versus chronic gastritis, suspicious for B-cell MALT malignant lymphoma. Immunoperoxidase studies showed that these atypical lymphoid cells were positive for CD20, CD79a, and CD43 and negative for CD5 (approximately 30% of positive cells), CD23, CD10, Cylcin D1, BCL6 (less than ten percent of cells were positive), Kappa and Lambda.
The duodenum had the same macroscopic findings, specifically in the duodenal bulb, first and second part of the duodenum. Biopsies of the duodenal bulb showed atypical lymphoid proliferation and active erosive chronic duodenitis. Colonoscopy showed angiectasia in the cecum and diffuse abnormal vascularity, erosions, granularity, and headed-up margins, again concerning for malignancy. Biopsy showed atypical lymphoid proliferation and erosions of surface mucosa.
Each biopsied specimen was sent out for a second opinion, and all specimens were diagnosed with EMZL-MALT. FISH analysis was positive for BIRC-3-MALT1 (11; 18) fusion, which is seen in approximately 50% of extranodal marginal zone B-cell lymphoma or mucosa associated lymphoid tissue type cases. In gastric MALTtype lymphomas, this fusion protein is a clonal marker for resistance to H. pylori eradication therapy, therefore considered antigen independent growth. Furthermore, the samples were negative for IGH-MALT1 (14;18) fusion and BCL6 rearrangement. Genetic results testing is seen in Table 1. A CT lung guided biopsy showed round cells consistent with lymphoid cells. The treatment plan was 8 cycles of rituximab/Bendamustine followed by rituximab alone (Table 1).
Table 1:Gene expression results of the biopsy taken from the patient.
Discussion
MALT lymphomas may arise from either lymphoid tissue (such as Peyer patches in the gut) or in organs that normally lack lymphoid tissue, but have accumulated B cells [3,5,8]. Immune system dysregulation caused by sustained stimulation from chronic infection and/or autoimmune process leads to this accumulation [3,5,7,8]. The most common site affected is the stomach in 60- 70% of cases followed by the small intestine, ileum, cecum, colon and rectum [8]. Extra-intestinal sites include salivary glands, lung, thyroid, skin, and ocular adnexa [3,5,7]. Patients with Sjogren syndrome have over a 40-fold increase risk of MZL-MALT involving the salivary glands [3,10]. Organisms such as Campylobacter jejuni have been implicated in MZL-MALT involving the small bowel while the Borrelia burgdorferi can cause MZL-MALT of the skin [3,8,10]. In up to 15% of cases the lung can be involved which is referred to as bronchus-associated lymphoid tissue (BALT) lymphoma [4]. It has been postulated that approximately a quarter of patients with gastric MALT lymphoma have disseminated, multiorgan involvement at presentation. The rate goes up to 50% in those with extra gastric MALT lymphomas [13].
Due to sustained auto-antigenic stimulation and subsequent polyclonal B cell proliferation, genotoxic reactive species are generated leading to leading to chromosomal instability, translocations, deletions, and trisomies [2,5-7]. The intrinsic genetic instability of B cells during somatic hypermutation and class-switch recombination also causes DNA damage. When genetic abnormalities accumulate, not only can histologic transformation occur, but loss of dependency from antigenic stimulation occurs which potentially leads to antibiotic resistance as in seen with our patient [14].
The most common chromosomal translocations with pathogenic significance include: t(11,18), t(14,18), t(1,14), and t(3,14) [1,7]. Among these genetic abnormalities, t(11, 18) (q21;q21) is the most frequently detected translocation in H. pylori - negative gastric MALT lymphoma [1,3]. In extra gastric lymphomas, particularly pulmonary MALT/ BALT lymphomas, it occurs in up to one third of cases [4,5,8,14]. At the genomic level, this translocation causes fusion of the apoptosis inhibitor 2-gene (AP12) with the MALT1 gene which generates a fusion protein, AP12-MALT1. This in turn activates NF-kB which regulates the expression of genes responsible for survival and proliferation of B cells [9,12]. The result is uncontrolled proliferation of B-cell clones and neoplastic transformation [9,12].
Multiple studies report significant association of t(11;18) (q21;q21) with advanced, disseminated disease particularly in gastric MALT lymphomas [5,9,13]. Interestingly, it has also been reported that t(11;18)(q21;q21) presents exclusive of other genetic abnormalities suggesting that this translocation is responsible for some form of genetic stabilization of the disease [9,13]. To further support this theory, Raderer et al. [13], also found patients with t(11;18)(q21;q21)- positive MALT lymphoma to have a significantly longer time to relapse (76 months) after complete remission compared with those lymphomas negative for this aberration (29 months). BCL 10 nuclear expression is another marker closely related to t(11;18) and is frequently found in disseminated gastric MALT lymphoma [6,9,11]. Trisomy 18 has been potentially linked to multiorgan disease in patients with extra-gastric MALT lymphoma; however, larger studies are needed [13].
Diagnosis is based on the histopathological evaluation of tissue biopsies and with an immunohistochemical panel. This panel should include at least the following markers: CD20, CD10, CD5 and cyclinD1, to help distinguish it from other indolent lymphomas [7,15]. In addition, FISH or reverse transcriptase PCR can be used to detect the presence of t(11;18)(p21;p21) and other chromosomal aberrations in order to identify those potentially unresponsive to antibiotic therapy [7,9]. Given the possibility of synchronous lesions along the GI tract, staging for MALT lymphomas should be meticulous and include gastroscopy, colonoscopy, bone marrow biopsy and CT of thorax and abdomen [8,10,13,14]. In BALT, tissue biopsy demonstrates polymorphous infiltrates of small lymphocytes, plasma cells, and B cells [4]. These may form characteristic lymphoepithelial lesions; however, often times histology and immunochemistry show no definitive features of a lymphoproliferative disease, as in our patient [4,16]. Recent studies show that the presence of monoclonal immunoglobulin heavy chain (IgH) gene rearrangement using PCR may be used to confirm BALT lymphomas [4,16]. This monoclonal B- cell population appears as a homogenous pattern, not a typical heterogeneous pattern which is seen in normal, polyclonal B- cell populations [4].
There is no consensus for the treatment of H. pylori negative MALT lymphoma nor for extra-gastric MALT lymphomas. First line treatment (even for H. pylori- negative gastric MALT lymphomas) is antibiotic therapy [1,7,8]. Even in absence of infection, there have been cases reporting response to antibiotic therapy [1,7,8]. One possible reason to this, aside from false negative H. pylori infection, is that another bacterium responsible for the lymphoma is susceptible to the antibiotic therapy [1,7].
Local radiotherapy (RT) has shown good disease control in those with early stage H. pylori-negative MALT lymphoma of the stomach, those with persistent lymphoma after antibiotic therapy, and those with localized non-gastric lymphomas [5,8,14]. Shrestha et al. [2] demonstrated successful treatment of EZML- MALT lymphoma with bronchial metastasis using RT. For patient who fail to achieve complete remission, or for those who present with more advanced disease, treatment with a single-agent chemotherapy, immunotherapy, or combination of both should be considered [5,8,14]. Common agents include rituximab, fludarabine, oral cyclophosphamide, chlorambucil, 2CdA (cladribine), and/or even R-CHOP/R-CNOP therapy [5,8,14]. Patients harboring t(11;18) translocation were found to be less likely to respond to oral alkylating agents [7,12]. Levy et al. [12] reported less than 10% of patients with t(11;18) translocation treated with oral alkylating agents remained in remission at long-term follow-up as compared to 89% of patients without t(11;18) [12]. Newer agents such as bendamustine, lenalidomide, and everolimus are currently undergoing clinical trials and may be promising in patients with refractory or advanced disease [5].
Extranodal marginal zone lymphomas of mucosa-associated lymphoid tissue can present in various ways and chromosomal abnormalities can influence multifocality and clinical presentation. Individuals found to have MALT lymphoma should have extensive staging and diagnostic work up looking for multi-organ involvement, particularly in those with t(11;18)(q21;q21) or trisomy 18. It has also been speculated that early systemic treatment could be beneficial in patients with t(11;18)(q21;q21) due to their potential of remaining dormant for a longer period of time before relapse and dissemination [9]. Because these lymphomas are rare and large studies are lacking, there is an urgent need for the establishment of clear management and treatment guidelines. This is particularly important in those with certain chromosomal abnormalities, disseminated disease, or advanced disease. This would enable clinicians to intervene earlier and tailor their treatment based on an individual’s presentation and genomic characteristics, leading to a decrease in morbidity and mortality (Figure 1).
Figure 1:Images of the stomach and duodenum showed diffuse continuous congestion, erythema, friability, granularity, and nodularity of the mucosa without bleeding.
References
- Asano N, Iijima K, Koike T, Imatani A, Shimosegawa T (2015) Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphomas: A review. World J Gastroenterol 21(26): 8014-8020.
- Shrestha B, Kim B, Huffstetler A (2016) An unusual presentation of gastric mucosa-associated lymphoid tissue (MALT)-type lymphoma. Journal of Community Hospital Internal Medicine Perspectives 6(4): 31707.
- Khalil MO, Morton LM, Devesa SS, Check DP, Curtis RE, et al. (2014) Incidence of marginal zone lymphoma in the United States, 2001-2009 with a focus on primary anatomic site. Br J Haematol 165(1): 67-77.
- Li P, Cheung L, Chiu B (2016) Early bronchus-associated lymphoid tissue lymphoma diagnosed with immunoglobulin heavy chain molecular testing. Canadian Respiratory Journal 2016: 7056035.
- Joshi M, Sheikh H, Abbi K, Sarah L, Kamal S, et al. (2012) Marginal zone lymphoma: Old, new, targeted, and epigenetic therapies. Therapeutic Advances in Hematology 3(5): 275-290.
- Liu H, Ye H, Dogan A, Ranaldi R, Hamoudi RA, et al. (2001) T(11;18) (q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 98(4): 1182- 1187.
- Zucca, Bergman C (2013) Gastric marginal zone lymphoma of MALT type: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 24(6): 144-148.
- Abbas H, Niazi M, Makker J (2017) Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma of the Colon: A case report and a literature review. Am J Case Rep 18: 491-497.
- Ye H, Liu H, Raderer M, Chott A, Ruskone Fourmestraux A, et al. (2003) High incidence of t(11;18) (q21;q21) in Helicobacter Pylori-negative gastric MALT lymphoma. Blood 101(7): 2547-2550.
- Raderer M, Vorbeck F, Formanek M, Osterreicher C, Valencak J, et al. (2000) Importance of extensive staging in patients with mucosaassociated lymphoid tissue (MALT)-type lymphoma. Br J Cancer 83(4): 454-457.
- Akoum R, Serhal W, Farhat H (2015) Disseminated gastric malt lymphoma with monoclonal gammopathy, t(11;18)(q21;q21), and subsequent development of t-large granular lymphocytic leukemia: A case report and review of the literature. Case Rep Med 2015: 953297.
- Lévy M, Copie Bergman C, Gameiro C, Chaumette MT, Delfau Larue MH, et al. (2005) Prognostic value of translocation t(11;18) in tumoral response of low-grade gastric lymphoma of mucosa-associated lymphoid tissue type to oral chemotherapy. J Clin Oncol 23(22): 5061-5066.
- Raderer M, Stefan W, Berthold S, Marlene T, Karl T, et al. (2006) Assessment of disease dissemination in gastric compared with extragastric mucosaassociated lymphoid tissue lymphoma using extensive staging: A singlecenter experience. Journal of Clinical Oncology 24(19): 3136-3141.
- Zinzani PL (2012) The many faces of marginal zone lymphoma. Hematology Am Soc Hematol Educ Program. 2012: 426-432.
- Periwal P, Khanna A, Dabral C, Talwar D (2016) A case of disseminated bronchus-associated lymphoid tissue lymphoma masquerading as nonresolving pneumonia. Lung India 33(5): 573-574.
- Goldman A, Bedolla G, Gebrail F, Cualing H (2003) Bronchus-associated lymphoid tissue lymphoma. Archives of Pathology & Laboratory Medicine 127(1): 115-116.
© 2018 Micaella Kantor. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






