- Submissions

Full Text
Gastroenterology Medicine & Research
Association between Non-Steroidal Anti- Inflammatory Drugs and The Risk of Recurrent Colorectal Adenomas: Renewed Meta-Analysis
Yin Wang*, Qian Zhang* and Tai Yun Zhao
The People’s Hospital of Bozhou, China
*Corresponding author: Yin Wang, The People’s Hospital of Bozhou, China Qian Zhang, The People’s Hospital of Bozhou, China
Submission: October 15, 2018 ;Published: November 13, 2018

ISSN 2637-7632Volume2 Issue2
Abstract
Aim: Previous studies reported uncertain results on the relationship between non-steroidal anti-inflammatory drugs (NSAIDS) and colorectal adenomas. We performed a renewed meta-analysis investigating the efficacy and safety of NSAIDS in preventing the colorectal adenoma recurrence.
Methods: Data were retrieved from the Medicine, Cochrane library, and Emase database for randomized controlled trials (RCTs) up to August 2018. Data, bias, and quality were extracted and assessed by two independent reviewers. The random-effects model of Stata 12.0 was used for data analysis.
Results: This study included 13 RCTs. Aspirin and rofecoxib/celecoxib decreased the risk of recurrent adenomas with risk ratios (RR) of 0.83 (95% confidence interval (CI), 0.74-0.93, P=0.248, I2=26%, high-quality) and 0.63 (0.58-0.68, P=0.8, I2=0% high-quality), respectively. Subgroup analysis of 4- and 5-year follow-up for adenoma recurrence, respectively, 1.02 (0.84-1.24, P=0.127, I2=51.5% moderate-quality); and 1.15 (0.88-1.50, P=0.026, I2=72.6% very low-quality) and advanced adenomas, respectively, 0.88 (0.68-1.14, P=0.479, I2=0% high-quality); and 1.16 ( 0.82 -1.63, P=0.364, I2=1.0% high-quality).
Conclusion: NSAIDS decreased risks of recurrent adenomas, and results of 1- and 3-year follow-up were similar, but long-term 4- or 5-year followup showed that NSAIDS did not prevent the recurrence of adenomas. However, long-term rofecoxib and celecoxib were related to serious cardiovascular disorders and major bleeding; therefore, we suggest that the duration of rofecoxib and celecoxib prophylactic treatment for adenoma recurrence should not be more than 3 years.
Keywords: Non-steroidal anti-inflammatory drugs; Colorectal adenoma; Aspirin; Celecoxib; Rofecoxib; Meta-analysis
What Does This Paper Add to the Literature?
Our study first reports the pros and cons of long-term oral NSAIDs, and most benefits were achieved with short-term treatment of patients. Especially, we identified that the duration of administration should be limited to not more than 3 years, which paves the way for further research.
Introduction
Colorectal carcinoma is one of the most common malignancies, accounting for the second most cancer-related deaths in the US. In 2016, approximately two million new cancer cases and nearly 60,000 new related deaths occurred in the US [1]. Although the morbidity and mortality in patients over 50 years has decreased because of the improvements in diagnosis and treatment, an investigation shows that in patients less than 50 years it will double by 2030, especially in those 20-34 years old [2]. Adenomas are known to be precursors of colorectal cancer, especially those with a large diameter (≥1cm), which are villous, tubulovillous, and highly malignant. Early detection and removal of the tumors through colonoscopy can significantly decrease the risk of colorectal carcinoma [3]. However, the strict bowel cleansing required before a colonoscopy, dietary changes before and after colonoscopy, uncomfortable treatment, and the relatively high cost result in a low level of compliance [4]. The recurrence rate of adenomas would be high even if all adenomas are removed [5,6] and continual screening increases the social and economic burden, making prevention a major public health goal.
Systematic reviews and meta-analyses [7-12] published to date indicate that non-steroidal anti-inflammatory drugs (NSAIDS) could prevent the recurrence of colorectal adenoma. Because of factors such as small sample sizes, high heterogeneity, and different baseline characteristics, these studies have not reached a consensus on the relationship between NSAIDS and the recurrence of colorectal adenoma. Therefore, we performed a systematic review and meta-analysis including the most recent published papers to confirm the efficacy and safety of NSAIDS in preventing the recurrence of adenomas in patients with different baseline characteristics.
Methods
Screening strategy and inclusion criteria
The Medicine, Cochrane library, and Embase databases were screened up to August, 2018, and we retrieved all RCTs that reported the efficacy and safety of NSAIDS, and the risk of recurrent colorectal adenomas using the following Mesh or keywords “non-steroidal anti-inflammatory drugs,” “adenoma*,” “aspirin,” “celecoxib,” and “rofecoxib,” and “random*.” The language of studies was restricted to English. We manually retrieved the missing literature from the references in relevant meta-analyses and systematic reviews.
Two reviewers (Yin Wang and Qian Zhang) checked the retrieved studies to identify the missing data. All papers included in our research were selected based on the following inclusion criteria:
1. RCTs comparing NSAIDS with a placebo;
2. trials enrolling patients who had undergone a colonoscopy and had polyps detected and resected;
3. the primary endpoint was the recurrent incidence of adenomas, advanced adenomas (defined by one or more of the following features: ≥1.0 cm, with villous or tubulovillous tissue architecture, or high-grade dysplasia), or both;
4. follow-up of at least 1 year.
The exclusion criteria were
1. RCTS without a placebo or treatment group;
2. Letters, reviews, comments, case reports, and studies with lost statistical data; and
3. Participants who had history of familial adenomatous polyposis (FAP) and inflammatory bowel disease (IBD).
Risk bias assessment
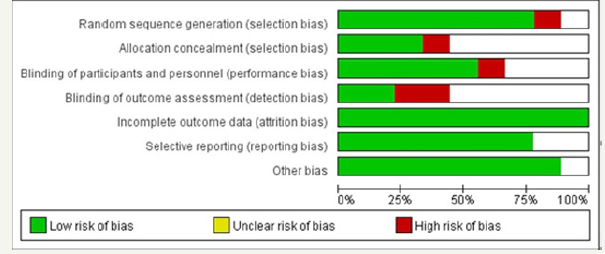
Two authors (Yin Wang and Qian Zhang) independently assessed the risk of bias with all RCTs based on the Cochrane risk of bias criteria using RevMan (version 5.1) [13,14], and each quality item was graded as low-, high-, or unclear-risk. The seven items used to evaluate bias in each trial included the randomization sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
Evidence grading
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to rate the quality of evidence of estimates (high, moderate, low, and very low) derived from meta-analyses using GRADE pro software. Reviewers independently assessed the confidence in effect estimates for all outcomes using the following categories: risk of bias, inconsistency, indirectness, imprecision, and publication bias [15,16].
Data extraction
Two researchers (Yin Wang and Qian Zhang) independently extracted the following data from every included trial: author, publication date, region, age, treatment duration, NSAIDS vs placebo, recurrent adenomas, and advanced adenomas. The third author made the final decision when there was any disagreement
Statistical analysis
The association between NSAIDS and incidences of recurrent colorectal adenomas was identified. We performed a meta-analysis of the risk ratios (RRs), and 95% confidence intervals (Cis) using the Mantel-Haenszel test statistic. The Stata 12.0 software was used to pool the data with a random-effects model and detect heterogeneity using the I2 statistic. The I2 value ranged from 0 to 100% (0–25%, no heterogeneity; 25–50%, moderate heterogeneity; 50-75%, extensive heterogeneity; and 75-100%, serious heterogeneity [13]. All meta-analyses were performed using Stata 12.0 (version 12.0; College Station, TX, USA) and RevMan 5.1 (version 5.1; The Nordic Cochrane Centre, Copenhagen, Denmark). GRADE profiler (McMaster University, Hamilton, ON, Canada) was used to create the summary of findings table. All tests were two-tailed and a P< 0.05 was considered statistically significant.
Results
Finally, 599 trials were finally screened, and 423 remained after duplicates were removed and 83 studies were identified after reading the titles and abstracts. The screening procedure yielded nine RCTS, reported in 13 publications (Figure 1). We considered eight publications accessing the same study at two different followup durations as a single study for all analyses. Figure 2 & 3 show the risk of bias assessments. All studies were clinical randomized double-blind controlled studies with high quality. Aspirin was administered in seven trials [17-23], rofecoxib in one [24], and celecoxib in the remaining [25-29]. Furthermore, low-dose aspirin (81mg/day) was included in two trials [18,19], 100mg/day in one trial [23], and 160mg/day in two trials, while high-dose aspirin (300mg/day) [20-22] and 325mg/day [17-19] was included in three trials each. Rofecoxib was administered at 25mg/day and celecoxib at 400 or 800mg/day, included in five [25-29] and two [25,26] trials, respectively. The follow-up times of these trials varied and included 1-5 years. The included trial characteristics are summarized in Table 1. The primary and secondary end points were the recurrence of any adenomas and advanced adenomas, and occurred in every trial..
Table 1:Characteristics of the included trials and participants.
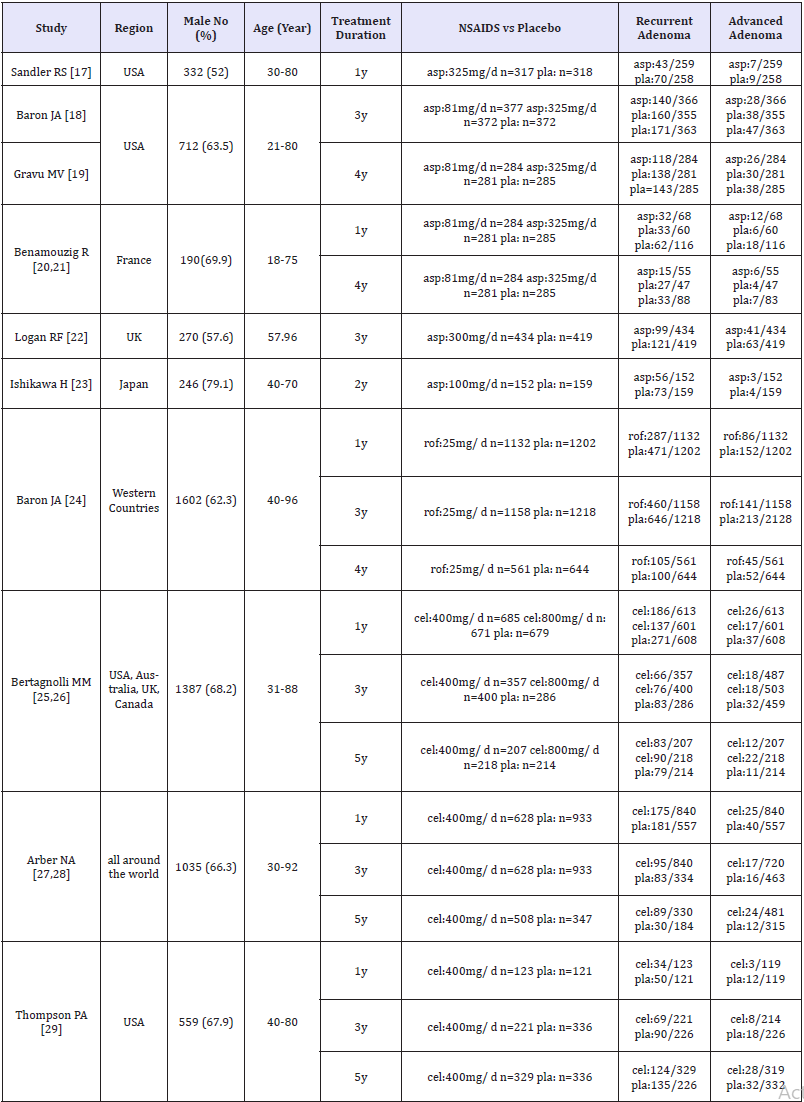
asp: Aspirin; pla: Placebo; rof: Rofecoxib; cel: Celecoxib
Figure 1:Study selection.
Figure 2:Bias risk map: a judgement of the percentage of all the projects that generate bias risk in the study.
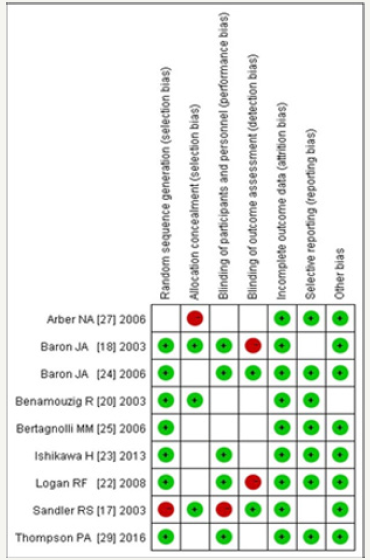
Figure 3:Bias risk map: the author’s judgement of each bias risk item in all studies.
Recurrence of adenomas or advanced adenomas
Nine trials compared NSAIDS with a placebo. As shown in Figure 4 & 5, NSAIDS significantly decreased the recurrence of colorectal adenomas with RR=0.72 (95%CI, 0.64- 0.82, p< 0.01, I2=71.6%) and advanced adenomas, RR=0.62 (95%CI, 0.53 to 0.72, P=0.403, I2=3.8%), for the high heterogeneity among the trials with the recurrence of adenomas. Subgroup analyses by the type of NSAIDS revealed that aspirin, rofecoxib, and celecoxib significantly decreased the recurrence of colorectal adenomas, with RR=0.83 (95%CI, 0.74-0.93, P=0.248, I2=26%, high-quality) for aspirin and RR=0.63 (95%CI, 0.58-0.68, P=0.8, I2=0% high-quality) for rofecoxib and celecoxib. While for the recurrence of advanced adenomas with low heterogeneity, the subgroup analysis by the type of NSAIDS revealed a significantly decreased in the recurrence of advanced adenomas with aspirin (RR=0.72 (95%CI, 0.57-0.90, P=0.767, I2=0%, high quality)) and rofecoxib and celecoxib (RR=0.54 (95%CI, 0.43- 0.68, P=0.335, I2=11.6% high-quality)) (Figure 4 & 5).
Figure 4:Random-effects model of RR of recurrence of adenomas to 1 and 2 vs placebo intervention (1: aspirin; 2: rofecoxib, celecoxib).
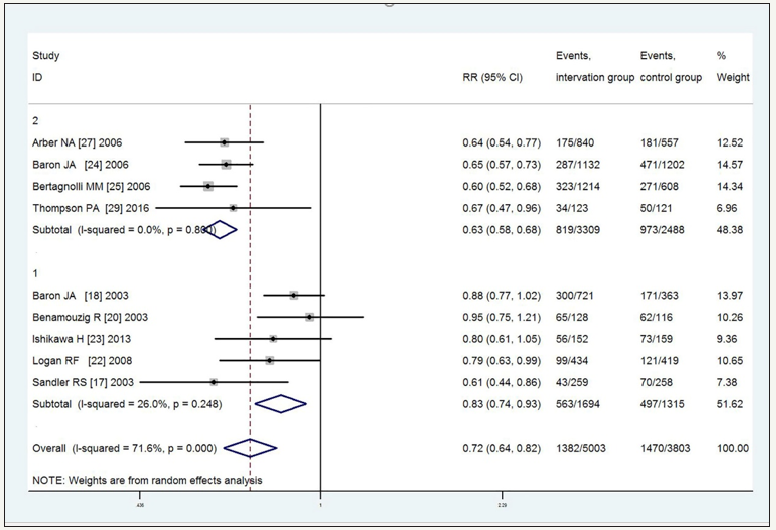
Figure 5:Random-effects model of RR of recurrence of advanced adenomas to 1 and 2 vs placebo intervention (1: aspirin; 2: rofecoxib, celecoxib).
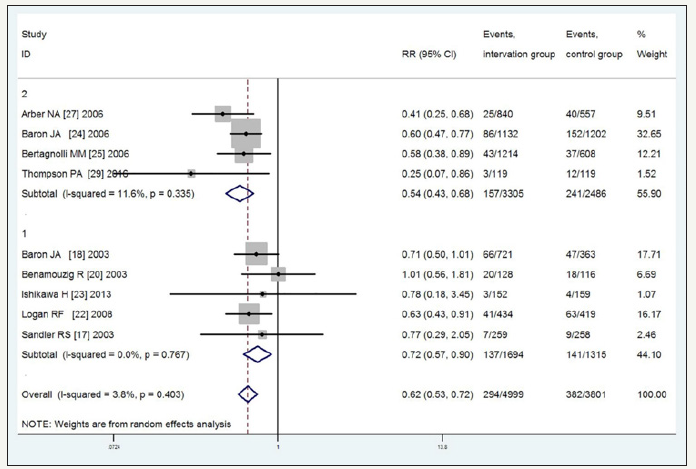
Figure 6:Incidence of recurrent adenomas with duration of follow-up time 1-year, 2-years, 3-years, 4-years and 5-years.
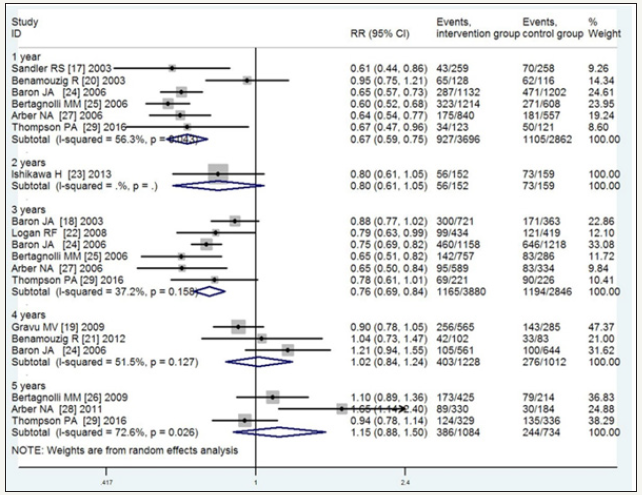
Subgroup analysis of the risk of recurrent adenomas based on 1-, 2-, 3-, 4-, and 5-year follow-up periods, revealed RR=0.67 (95%CI, 0.59-0.75, P=0.043, I2=56.3% moderate-quality), 0.80 (95%CI, 0.61-1.05, low-quality), 0.76 (95%CI, 0.69-0.84, P=0.158, I2=37.2% high-quality), 1.02 (95%CI, 0.84-1.24, P=0.127, I2=51.5% moderatequality), and 1.15 (95%CI, 0.88-1.50, P=0.026, I2=72.6% very lowquality), respectively. The corresponding values for advanced adenomas were 0.58 (95%CI, 0.46-0.73, P=0.295, I2=18.3% highquality), 0.78 (95%CI, 0.18-3.45, P=0.335, I2=11.6% low-quality), 0.66 (95%CI, 0.57-0.76, P=0.816, I2=0% high-quality), 0.88 (95%CI, 0.68-1.14, P=0.479, I2=0% high-quality), and 1.16 (95%CI, 0.82- 1.63, P=0.364, I2=1.0% high-quality). These results indicated that with the extension of the follow-up, the efficacy of the NSAIDS in preventing the recurrence of adenomas and advanced adenomas gradually decreased, especially with the 4- and 5-year follow-up, and no significant interactions were observed between the NSAIDS and placebo. As shown in Figure 6 & 7, the significant heterogeneity in the recurrence of adenomas with the 1-, 4-, and 5-year follow-up was explainable by the type of NSAIDS. For the small sample, the analytical result of the 2-year follow-up was not precise (Figure 6 & 7).
Figure 7:Incidence of recurrent advanced adenomas with duration of follow-up time 1-year, 2-years, 3-years, 4-years and 5-years.
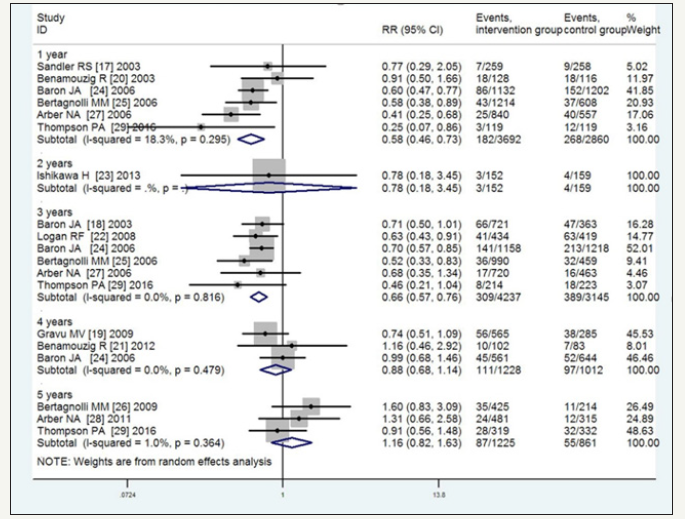
Table 2:Summary of finding table about the quality of evidence of estimates*. The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
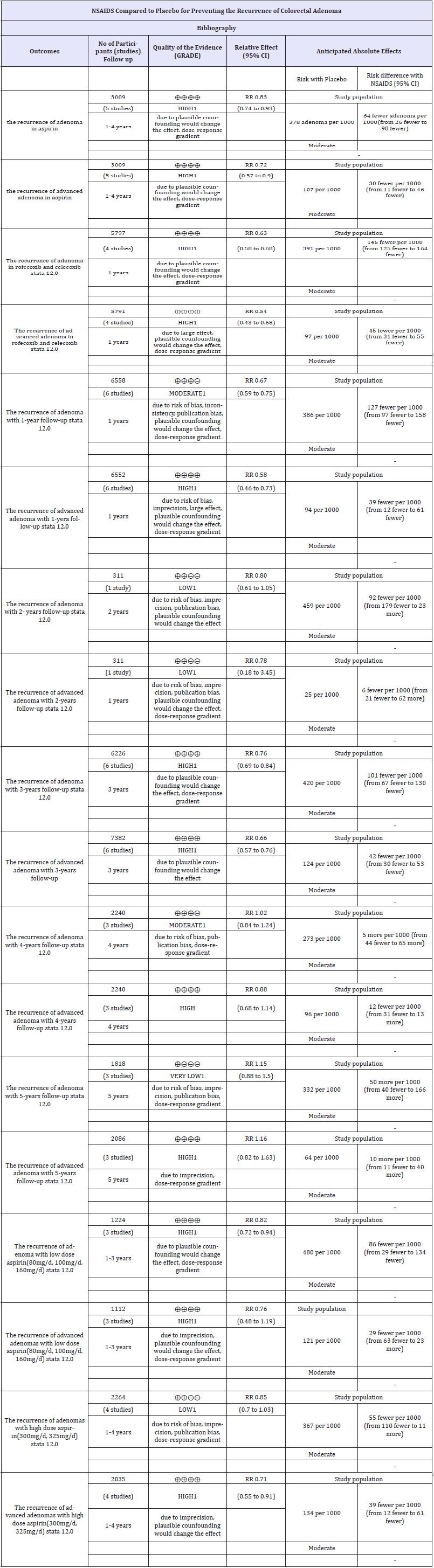
CI: Confidence Interval; RR: Risk Ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. 1 No explanation was provided.
For low-dose aspirin (80, 100, and 160mg/day), the RR of the recurrence of any adenoma among three trials was 0.82 (95%CI, 0.72-0.94, P=0.88, I2=0% high-quality), whereas the RR for the recurrence of advanced adenoma was 0.76 (95%CI, 0.48-1.19, P=0.275, I2=22.6% high-quality). For high-dose aspirin (300 and 325mg/day), the RR for the recurrence of any adenoma among four trials was 0.85 (95%CI, 0.70-1.03, P=0.05, I2=61.5% moderatequality), whereas the RR for the recurrence of advanced adenoma was 0.71 (95%CI, 0.55-0.91, P=0.789, I2=0% high-quality). The quality of the evidence of estimates was calculated using GRADE pro software and is shown in Table 2.
Adverse events
Table 3:The incidence of adverse events comparing COX-1 andCOX-2 with placebo.
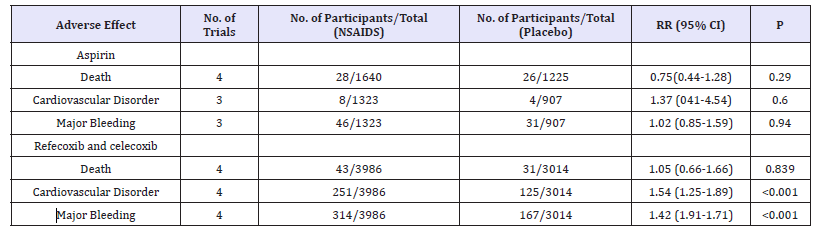
For adverse events, several trials were reported in the metaanalysis, including death, myocardial infarction, and bleeding. For aspirin, the meta-analysis showed no significant differences compared with the placebo in death, myocardial infarction, and bleeding with RR of 0.75 (95%CI, 0.44-1.28, P=0.29), 1.37 (95%CI, 0.41-4.54, P=0.6), and 1.02 (95%CI, 0.85-1.59, Ph 0.94), respectively. While the incidences of death, myocardial infarction, and bleeding with rofecoxib and celecoxib compared with the placebo showed RRs of 1.05 (95%CI, 0.66-1.66, P=0.839), 1.54 (95%CI, 1.25-1.89, P< 0.001), and 1.42 (95%CI, 1.19-1.71, P< 0.001), and the results indicated that cyclooxygenase (COX)-2 may increase the risk of myocardial infarction and bleeding compared to the placebo (Table 3).
Discussion
This renewed meta-analysis included all high-quality RCTs and showed that aspirin, rofecoxib, and celecoxib could decrease the recurrence of adenomas and advanced adenomas. In addition, and the quality of evidence of estimates was high using GRADE pro software. Five subgroups with 1-, 2-, 3-, 4-, and 5-year followup, these results suggest that with the extension of follow-up, the NSAIDS showed less efficacy. Furthermore, there was no significant association of oral NSAIDS with decreased recurrence of adenomas and advanced adenomas with the 4- and 5-year follow-up. The quality of evidence of estimates was not consistent with that of a meta-analysis by Yin Wang et al. [9] reported that oral NSAIDS were significantly associated with increased risk recurrence of adenomas with follow-up >3 years. However, for the advanced adenomas, the results are consistent. Although the heterogeneities were significant with the >3-year follow-up in the two meta-analyses, the sample size of the 4- and 5-year follow-up of this current meta-analysis was almost three times that of the former metaanalysis, which indicates the strength, credibility, and accuracy of the results. However, higher quality randomized trials with longer follow-up durations comparing NSAIDS vs placebo are still needed to conclude our results.
Random-effects were used to pool data for the low-dose aspirin since randomization indicated a significant reduction in the risk of recurrent adenomas, and the quality of evidence of estimate was high. However, low-dose aspirin had no effect on recurrence of advanced adenomas, and the quality of evidence of estimate was high. This result was different from that of the study by Veettil et al. [11] who thought that low-dose aspirin had slight preventative effects on the recurrence of advanced adenomas. Information on prevention of recurrence of adenomas by high-dose aspirin showed it had no significant efficacy with substantial heterogeneity; however, a significant reduction was identified in the recurrence of advanced adenomas with no heterogeneity, and the quality of evidence of estimate was high. These results are consistent with those of Veettil et al [11]. However, because of the significant heterogeneity and limited sample size, additional studies with large sample sizes and high-quality randomized controlled trials should be designed to confirm the results.
The included studies reported on death, cardiovascular disorders, and bleeding events. The results comparing the aspirin and placebo groups were similar, to those of rofecoxib and celecoxib, in death, and no significant difference was observed between COX-2 and the placebo. However, the incidence of cardiovascular disorders and bleeding events were significantly higher in the rofecoxib and celecoxib groups than in the control group. The pooled summary indicated no significant reductions in the recurrence of adenomas and advanced adenomas for the 4- and 5-year follow-up, especially, the subgroup with the 5-year follow-up including participants who were all administered rofecoxib and celecoxib. From the above statistical analysis, long-term oral administration of rofecoxib and celecoxib did not significantly prevent the recurrence of colorectal adenomas and advanced adenomas. Furthermore cardiovascular disorders and bleeding events caused by long-term oral administration of rofecoxib and celecoxib should be seriously considered as well as the appropriate duration for these agents that would provide the most benefits to patients. Therefore, future research studies should focus on these aspects.
Our research has numerous advantages. All RCTs that validated the benefits of NSAIDS in preventing the risk of recurrent colorectal adenomas were screened. The studies were well conducted with high compliance, high follow-up rates, and longer duration of time. The sample size in this meta-analysis was larger than that of the former. Thus, this analysis has high validity. Especially, the follow-up time in the included trials was sufficient to analyze the long-term effects of NSAIDS on the risk of recurrent adenomas and advanced adenomas, and this discovery has filled a knowledge gap in the field.
However, this analysis also has some shortcomings. First, with the 2-year follow-up, only one study showed aspirin have no efficacy on preventing the recurrence of adenomas and advanced adenomas, and the small sample size restricted the accuracy of the result. Second, our findings on the follow-up-response patterns are not convincing because of the high heterogeneity and small sample size and, therefore, the type and dose of NSAIDS may have an effect. Third, because of the low number of trials or insufficient sample size, we were unable to assess the dose-response of aspirin in preventing the incidence of recurrent and advanced adenoma.
In summary, this meta-analysis demonstrated that aspirin, rofecoxib, and celecoxib decreased the incidence of recurrent colorectal and advanced adenomas, and the quality of evidence of estimates was high. NSAIDS can significantly decrease the recurrence of adenomas and advanced adenomas with a 1- and 3-year followup, but with the long-term follow-up of 4- or 5-years, they have no preventative effects. Furthermore, long-term oral rofecoxib and celecoxib were associated with important cardiovascular disorders and major bleeding and, therefore, we suggest that the duration of oral rofecoxib and celecoxib for preventing adenomas should be restricted to no more than 3 years. However, because of the limited sample size and significant heterogeneity, the safety of long-term rofecoxib and celecoxib preventative treatment should be included in future considerations of the risks and benefits.,
Author Contributions
Yin Wang: data collection, extraction, and analysis; writing the article. Qian Zhang: data analysis and modifying the article. Yao-Jun Wang: technology and method guidance, and handle differences.
References
- Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2018. CA Cancer J Clin 66(1): 7-30.
- Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez Bigas MA, et al. (2014) Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 150(1): 17-22.
- Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, et al. (2008) Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 58(3): 130-160.
- Seeff LC, Royalty J, Helsel WE, Kammerer WG, Boehm JE, et al. (2013) Clinical outcomes from the CDC’s Colorectal Cancer Screening Demonstration Program. Cancer 119 Suppl 15: 2820-2833.
- Bonithon Kopp C, Piard F, Fenger C, Cabeza E, O’Morain C, et al. (2004) Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum 47(3): 323-333.
- Cottet V, Jooste V, Fournel I, Bouvier AM, Faivre J, et al. (2012) Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut 61(8):1180-1186.
- Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, et al. (2009) Aspirin for the chemoprevention of colorectal adenomas: Meta-analysis of the randomized trials. J Natl Cancer Inst 101(4): 256-266.
- Gao F, Liao C, Liu L, Tan A, Cao Y, et al. (2009) The effect of aspirin in the recurrence of colorectal adenomas: a meta-analysis of randomized controlled trials. Colorectal Dis 11(9): 893-901.
- Wang Y, Zhang FC, Wang YJ (2015) The efficacy and safety of non-steroidal anti-inflammatory drugs in preventing the recurrence of colorectal adenoma: a meta-analysis and systematic review of randomized trials. Colorectal Dis 17(3): 188-196.
- Zhao TY, Tu J, Wang Y, Cheng DW, Gao XK, et al. (2016) The efficacy of aspirin in preventing the recurrence of colorectal adenoma: A renewed meta-analysis of randomized trials. Asian Pac J Cancer Prev 17(5): 2711- 2717.
- Veettil SK, Teerawattanapong N, Ching SM, Lim KG, Saokaew S, et al. (2017) Effects of chemopreventive agents on the incidence of recurrent colorectal adenomas: a systematic review with network meta-analysis of randomized controlled trials. OncoTargets and Therapy 10: 2689-2700.
- Veettil SK, Lim KG, Ching SM, Saokaew S, Phisalprapa P, et al. (2017) Effects of aspirin and non-aspirin nonsteroidal anti-inflammatory drugs on the incidence of recurrent colorectal adenomas: a systematic review with meta-analysis and trial sequential analysis of randomized clinical trials. BMC Cancer 17(1): 763.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, et al. (2011) The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343: d5928.
- Akl EA, Sun X, Busse JW, Johnston BC, Briel M, et al. (2012) Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol 65(3): 262- 267.
- Atkins D, Best D, Briss PA (2004) Grading quality of evidence and strength of recommendations. BMJ 328(7454): 1490.
- Guyatt GH, Oxman AD, Vist GE, Regina K, Yngve FY, et al. (2008) GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650): 924-926.
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, et al. (2003) A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 348(10): 883-890.
- Baron JA, Cole BF, Sandler RS, Robert WH, Dennis A, et al. (2003) A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 348: 891-899.
- Grau MV, Sandler RS, McKeown Eyssen G, Bresalier RS, Haile RW, et al. (2009) Nonsteroidal anti-inflammatory drug use after 3 years of aspirin use and colorectal adenoma risk: Observational follow-up of a randomized study. J Natl Cancer Inst 101: 267-276.
- Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, et al. (2003) Daily soluble aspirin and prevention of colorectal Adenoma recurrence: Oneyear results of the APACC Trial. Gastroenterology 125(2): 328-336.
- Benamouzig R, Uzzan B, Deyra J, Martin A, Girard B, et al. (2012) Prevention by daily soluble aspirin of colorectal adenoma recurrence: 4-year results of the APACC randomised trial. Gut 61(2): 255-261.
- Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR, et al. (2008) Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology 134(1): 29-38.
- Ishikawa H, Mutoh M, Suzuki S, Tokudome S, Saida Y, et al. (2014) The preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut 63(11): 1755-1759.
- Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, et al. (2006) A randomized trial of rofecoxib for the Chemoprevention of colorectal adenomas. Gastroenterology 131(6): 1674-1682.
- Bertagnolli MM, Eagle CJ, Zauber AG, Mark R, Scott DS, et al. (2006) Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 355: 873-884.
- Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Breazna A, et al. (2009) Five-year efficacy and safety analysis of the adenoma prevention with celecoxib trial. Cancer Prev Res 2(4): 310-321.
- Arber N, Eagle CJ, Spicak J, István R, Petr D, et al. (2006) Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 355: 885- 895.
- Arber N, Spicak J, Rácz I, Zavoral M, Breazna A, et al. (2011) Five-year analysis of the prevention of colorectal sporadic adenomatous polyps trial. Am J Gastroenterol 106(6): 1135-1146.
- Thompson PA, Ashbeck EL, Roe DJ, Fales L, Buckmeier J, et al. (2016) Celecoxib for the prevention of colorectal adenomas: Results of a suspended randomized controlled trial. J Natl Cancer Inst 108(12): 3-11.
© 2018 Yin Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






