- Submissions

Full Text
Gastroenterology Medicine & Research
Interferon-Free Therapy for Hepatits C in Brazil and Sustained Virological Response
Jonathan Soldera1,2*, Rafael Sartori Balbinot1, Ana Laura Facco Muscope1, Raul Angelo Balbinot1,3 and Silvana Sartori Balbinot1,3
1Universidade de Caxias do Sul, Brazil
2Department of Gastroenterology and Hepatology, Universidade Federal de Ciencias da Saude de Porto Alegre, Brazil
3Universidade de Sao Paulo, Braz
*Corresponding author: Jonathan Soldera, Professor of Gastroenterology and Hepatology, Faculty of Medicine, Universidade de Caxias do Sul, Ver Mario Pezzi Av, 699/601, Caxias do Sul - RS, CEP 95084-180, Brazil
Submission: October 28, 2017; Published: December 15, 2017

ISSN 2637-7632
Volume1 Issue2
Abstract
Introduction: Hepatitis C has been treated with interferon and ribavirin for over a decade with described global sustained virological response rates of 33% to 56%. Direct acting antiviral drugs available since 2013 in USA and 2015 in Brazil are changing this reality.
Purpose: Analyze the real-life efficacy and safety of interferon-free therapy.
Methods: Repot six cases of different treatments guided by north-american and european guildelines.
Results: Every reported patient achieved sustained virological response. The only adverse event was anemia in one patient.
Conclusion: Direct-acting antiviral drugs will dramatically change the population which can be treated and increase sustained virological response rates.
Keywords: Brazil; Chronic hepatitis C; Direct antiviral therapy; HCV; Real World
Introduction
Chronic hepatitis C (HCV) is one of the most common liver diseases, affecting around 185 million people worldwide [1] and it can evolve into cirrhosis and hepatocellular carcinoma. In Brazil, it is estimated a prevalence of 1.23%, found among blood donors with positive serology [2], and that 3.9 to 7.6 million people are chronically infected [3].
For about two decades, the treatment of HCV was based in interferon and ribavirin regimens. Although it has been used until recently, only 33% to 43% achieved sustained virological response [4]. With the introduction of pegylated interferon, SVR increased to a peak of 54% to 56% [5].
The introduction since 2013 in USA and since 2015 in Brazil of direct-acting antiviral drugs (DAAD) sofosbuvir, daclastasvir, simeprevir and ledipasvir has increased SVR rates and reduced adverse events associated with treatment dramatically [6].
This paper aims to report six cases of patients treated with DAAD between 2015 and 2016 based in guidelines from American Association for Study of the Liver (AASLD) and European Association for Study of the Liver (EASL) and review the literature regarding this topic.
Case Report
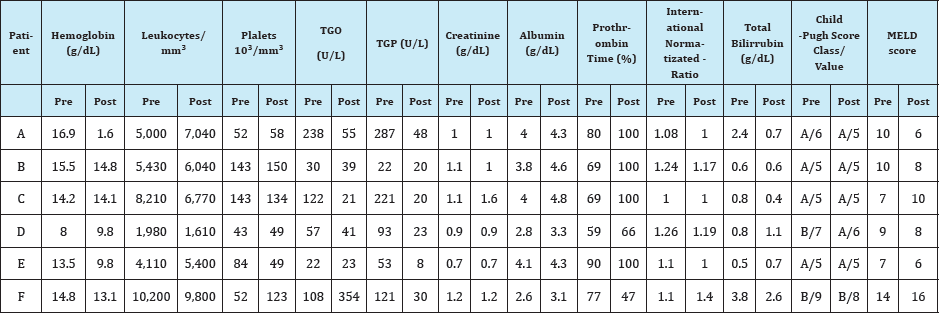
The authors report six cases of treatment that started and were concluded between June, 2015 and May, 2016, all prescribed in the first semester of 2015. The laboratorial data before and after treatment are summarized on Table 1.
Patient A
Patient: Male, 49 years old, cirrhosis due to HCV genotype 1b and alcohol abuse.
Clinical condition pre-treatment: Asymptomatic with no previous decompensation.
Treatment: Simeprevir 150mg once a day and sofosbuvir 400mg once a day for 24 weeks.
Complications: None.
Outcome: Sustained virological response.
Table 1: Summarized laboratorial data for the six reported cases pre and post-treatment.
Patient B
Patient: Male, 49-years old, HCV genotype 1b, liver biopsy dated to 2014 METAVIR A1F3.
Clinical condition pre-treatment: Asymptomatic.
Treatment: Sofosbuvir 400mg once a day and simeprevir 150mg once a day for 12 weeks.
Complications: None.
After treatment, an ultrasonography showed heterogeneous liver and an upper digestive endoscopy small esophageal varices.
Outcome: Sustained virological response.
Patient C
Patient: Male, 49-years old, cirrhosis due to HCV genotype 3, previous treatment with pegylated-interferon without SVR before renal transplantation.
Clinical condition pre-treatment: No previous decompensation, renal transplantation 7 years ago.
Treatment: Sofosbuvir 400mg once a day, daclatasvir 60mg once a day and ribavirin 1.25 grams daily for 24 weeks.
Complications: Patient presented with anemia which coincided with worsening of kidney function in week 8 being managed with reduction of ribavirin dosage to 750mg daily and erythropoietin (EPO). The lowest hemoglobin level was on week 12 of 7g/dL, motivating blood transfusion and suspension of ribavirin.
Outcomes: Sustained virological response.
Patient D
Patient: Female, 73-years old, cirrhosis due to HCV genotype 1b.
Clinical condition pre-treatment: Previous esophageal variceal bleeding (currently in endoscopic variceal ligation program), previous spontaneous bacterial peritonitis, and previous hepatorenal syndrome responsive to terlipressin and albumin.
Complications: None.
Treatment: Sofosbuvir 400mg + Ledipasvir 90mg once a day and ribavirin 1g/day for 12 weeks. She received EPO weekly during the treatment.
Outcome: Sustained virological response.
Patient E
Patient: Female, 70-years old, cirrhosis due to HCV genotype 1a.
Clinical condition pre-treatment: Child-Pugh A and MELD 6 previous to treatment, with no previous decompensation of cirrhosis.
Treatment: Sofosbuvir 400mg once a day, simeprevir 150mg once a day and ribavirin 1g/day for 24 weeks.
Complications: None.
Outcome: Sustained virological response.
Patient F
Patient: Male, 67-years old, cirrhosis due to HCV genotype 1b, failed to previous treatment with interferon and ribavirin.
Clinical condition pre-treatment: Mild obesity, type 2 diabetes. Previous spontaneous bacterial empyema, hepatic encephalopathy, ascitis, hepatic hydrothorax. He was listed for liver transplantation.
Treatment: Sofosbuvir 400mg once a day, daclatasvir 60mg once a day and ribavirin 1.25g/day for 12 weeks.
Complications: On week 4, he presented with pneumonia due to a multi-resistant Serratia marcescens with worsening of hepatic hydrothorax and a hepatorenal syndrome that responded to terlipress in plus albumin, managed with meropenem and thoracocentesis. Treatment was not interrupted and doses were not reduced.
Outcome: Sustained virological response.
Discussion
The goal for HCV treatment has always been one: achievement of sustained virological response, which increases quality of life and reduces morbidity and mortality. Among cirrhotic patients, it has been associated to lower MELD score and better liver function. The better understanding of the viral genome and its proteins has allowed the development of DAAD, which are close to the ideal drug - high SVR rates, taken once daily, lower side-effects and shorter period of treatment [6].
Sofosbuvir (SOF) is a nucleotide analog inhibitor of the NS5B polymerase of pangenotipic action and is highly tolerated with few side-effects [7]. Simeprevir (SIM) is a NS3/4 protease inhibitor of second generation used only for genotype 1 [8]. Daclatasvir (DCL) is a NS5A polymerase inhibitor of pangenotipic action [9].
Patients with genotype 1 naive treated with SOF and SIM have achieved SVR of 93 to 95% [8,10] and treated with SOF and DCL reached SVR around 98% in 12-week-treatment in naive patients and 100% [9] in 24-week-treatments in experimented patients. In genotype 3, it has been achieved a SVR of 90% and 86% for naive and experimented patients, respectively, with a 12 week regimen [11]. It has been described a prevalence of 1.8 to 8% of HCV infection in kidney transplantation recipients, most of them infected pre-transplantation. Hence, it's important to test these patients for HCV before and after kidney transplantation [12].
Patient D had her treatment chosen based on SOLAR-2 study, which accessed efficacy of SOF and ledipasvir association for decompensated cirrhosis for genotypes 1 and 4, with high SVRs and improvement in liver function [13].
Patient F was treated with SOF plus DCL plus RBV for 12 weeks according to study ALLY-1, which showed a 100% SVR rate on patients with genotype 1b and decompensated cirrhosis [14]. Data from a Brazilian cohort of 219 patients has been published recently, achieving high SVR [15]. These treatments were prescribed in 2015, before there was a Brazilian clinical protocol for treatment of HCV. Later, such was published, and recommended treatments were different than those who were used based on north-american and European guidelines available in that period of time.
Conclusion
Therapeutic regimens for HCV treatment with DAA are bound to change the natural history of HCV infection, since it is allowing treatment for patients who would otherwise be referred for liver transplantation: decompensated cirrhosis and post-kidney transplantation.
References
- Lavanchy D (2014) Envolving epidemiology of hepatitis C virus. Clin Microbiol Infect 17(2): 107-115.
- Fonseca J (1999) Epidemiologia da infecfao pelo virus da hepatite C no Brasil. Relatorio do Grupo de Estudo da Sociedade Brasileira de Hepatologia GED 18(Suppl 1): S3-S8.
- World Health Organization (2000) Hepatitis C - global prevalence (update). Wkly Epidemiol Rec 75: 17-28.
- Kjaergard LL, Krogsgaard K, Gluud C (2001) Interferon alfa with or without ribarivin for chronic hepatitis C: systematic review of randomized trials. BMJ 323(7322):1151-1155.
- Kohli A, Shaffer A, Sherman A, Kottilil S (2014) Treatment of hepatitis C: a systematic review. JAMA 312(6):631-640.
- Schinazi R, Halfon P, Marcellin P, Asselah T (2014) HCV direct-acting antiviral agentes: the best interferon free combinations. Liver Int 34(Suppl 1): 69-78.
- Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, et al. (2013) Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment- naive patients with hepatitis C genotype-1 infection (ATOMIC): an open- label, randomised, multicentre phase 2 trial. Lancet 381(9883): 21002107.
- Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-TM, Younossi ZM, et al. (2014) Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMO randomised study. Lancet 384(9956): 1756-1765.
- Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, et al. (2015) Alloral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 61(4): 1127-1135.
- Pearlman BL, Ehleben C, Perrys M (2014) The Combination of Simeprevir and Sofosbuvir is more effective than that of Peginterferon, Ribavirin, and Sofosbuvir for Patients With Hepatitis C-Related Child's Class A Cirrhosis. Gastroenterology 148(4): 762-770.
- Leroy V, Angus P, Bronowicki JP, Dore GJ, Hezode C, et al. (2016) Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: A randomized phase III study (ALLY-3+). Hepatology 63(5): 1430-1441.
- Scott DR, Wong JK, Spicer TS, Dent H, Mensah FK, et al. (2010) Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation 90(11): 1165-1171.
- Manns M, Samuel D, Gane EJ, Mutimer D, McCaughanm BM, et al. (2016) Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 16(6): 685-697.
- Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, et al. (2016) Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology 63(5): 1493-1505.
- Cheinquer H, Sette H, Wolff FH, de Araujo A, Coelho BS, et al. (2017) Treatment of Chronic HCV Infection with the New Direct Acting Antivirals (DAA): First Report of a Real World Experience in Southern Brazil. Ann Hepatol 16(5): 727-733.
© 2017 Jonathan Soldera, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






