- Submissions

Full Text
Gastroenterology Medicine & Research
Safety of Everolimus in Living Donor Liver Transplantation Recipients with Severe Renal Dysfunction with Low Estimated Glomerular Filtration Rate: Can Everolimus Help in Renal Recovery?
Ashok Thorat, Long-Bin Jeng*, Horng-Ren Yang
Organ Transplantation Center, China Medical University Hospital, Taichung, Taiwan
*Corresponding author: Long-Bin Jeng, Organ Transplantation Center, China Medical University Hospital, 2, Yuh-Der Road, Taichung, 40447, Taiwan
Submission: December 07, 2017; Published: December 15, 2017

ISSN 2637-7632
Volume1 Issue1
To Editor
Chronic renal dysfunction is a frequent and severe complication in solid-organ transplant recipients. Switching from calcineurin inhibitors (CNIs) to non-nephrotoxic mammalian target of rapamycine inhibitors (mTORi) such as everolimus (EVR) can improve renal function in these patients. Single center prospective study by Castroagudin et al. [1] has shown an improvement of renal function after addition of the EVR to primary immunosuppression and reducing the CNIs [1]. The conclusion of the recently conducted global clinical randomized trial H23O4 involving the EVR was that the introduction of EVR with tacrolimus (TAC) reduction from day 30 after liver transplantation achieved a superior renal function with no compromise in efficacy at 12 months after liver transplantation. The safety profile of EVR + Reduced TAC presented no unexpected safety concerns and showed similar tolerability to the standard tacrolimus regimen [2]. However, very few reports exist to show the safety and efficacy of EVR when used at early stage after living donor liver transplantation (LDLT) [3,4].
Table 1: Serum creatinine and eGFR values of the study cohort.
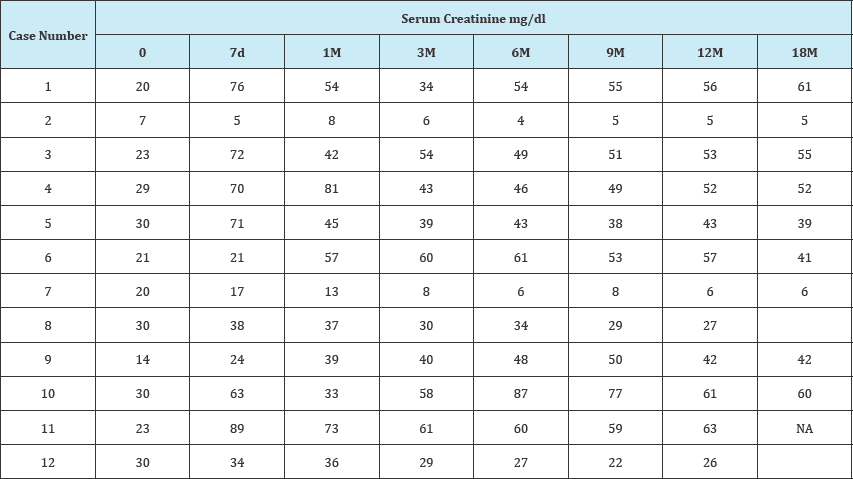
Table 1B:
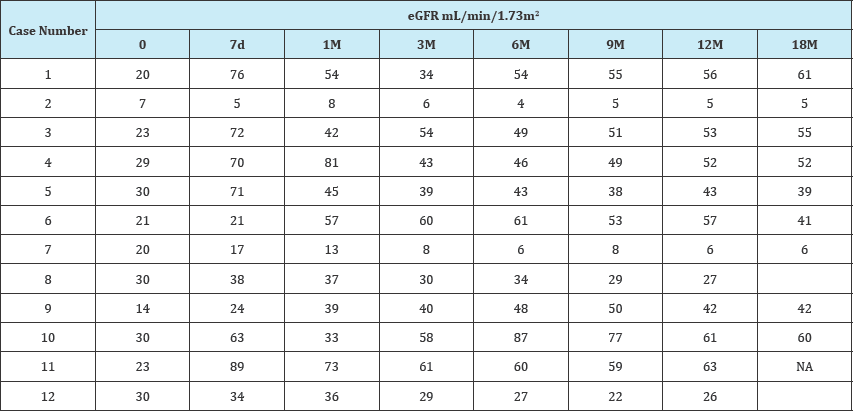
From January 2012 till October 2014, 215 recipients (Male:Female, 166:49; age, 54±10 years) that underwent LDLT received TAC-EVR based primary immunosuppression within 1st month of transplantation (4th to 20th day after LDLT) with minimum 12 months of follow up were retrospectively studied to evaluate the impact of EVR based primary immunosuppression on the course of renal functions in post-transplant period. 27 patients had prior renal dysfunction with serum creatinine >1.5mg/dl and the estimated glomerular filtration rate (eGFR) <60mL/min/1.73m2. Twelve of the recipients (n=12) from this subgroup with eGFR<30 were further evaluated and postoperative laboratory records were assessed. 66.6% (8/12) patients showed improvement in the renal functions. The average serum creatinine levels in these patients at pre-transplant, 1 month, 6 month and 12 months post-transplant were 2.68, 1.36, 1.26, and 1.29mg/dl, respectively (Table 1). The eGFR values at same time period were 23.75, 52.85, 56, and 53.37mL/min/1.73m2, respectively (Table 2). In four patients the renal functions deteriorated further requiring haemodialysis. The laboratory values of the study cohort are shown in Table 1. Average TAC trough levels at 7day, 3 month and 12 month post-transplant were 3.88±2.54ng/ml, 6.53±6.46ng/ml and 3.64±1.37ng/ ml, respectively. The EVR trough levels at same period were 3.54±1.54ng/ml, 3.7±1.04ng/ml, and 4.04±1.70ng/ml, respectively.
The effect of EVR-reduced TAC combination on the renal functions has been reported in our earlier study [3]. In this study the renal functions improved in 66.6 % of the recipients with prior renal failure. Fischer et al demonstrated that an early conversion from a CNI-based to an EVR-based regimen can be achieved safely, with beneficial effects on renal function [5]. In their study, the incidence of significant proteinuria was low overall, although, in the EVR plus reduced TAC group showed higher proteinuria than the standard TAC group (3.7% vs 0.8%, respectively; P=0.063), and proteinuria was the leading cause of study drug discontinuation (eight vs one patient). However, in our study significant proteinuria was nil.
In conclusion, the addition of the mTOR inhibitor-based immunosuppression improves kidney function even in patients with severe pre-transplant renal insufficiency. The safety and tolerability of the immunosuppression was unaffected even if EVR was started in first postoperative week after LDLT. Role of EVR in LDLT recipients with severe renal failure should be further studied to strengthen this finding.
References
- Castroagudín JF, Molina E, Romero R, Otero E, Tome S, et al. (2009) Improvement of renal function after the switch from a calcineurin inhibitor to everolimus in liver transplant recipients with chronic renal dysfunction. Liver Transpl 15(12): 1792-1797.
- De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, et al. (2012) Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant 12(11): 3008-3020.
- Jeng LB, Thorat A, Hsieh YW, Yang HR, Yeh CC, et al. (2014) Experience of using everolimus in the early stage of living donor liver transplantation. Transpl Proc 46(3): 744-748.
- Masetti M, Montalti R, Rompianesi G, Codeluppi M, Gerring R, et al. (2010) Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am J Transplant 10(10): 2252-2262.
- Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, et al. (2012) A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation - PROTECT. Am J Transplant 12(7): 1855-1865.
© 2017 Ashok Thorat, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






