- Submissions

Full Text
Gastroenterology Medicine & Research
Liver Fibrosis: Difficulties in Diagnostic and Treatment: A Review
Florian Bert*
Department of Internal Medicine, Krankenhaus Nordwest, Germany
*Corresponding author: Florian Bert, Department of Internal Medicine, Krankenhaus Nordwest, Steinbacher Hohl 2-26, 60488 Frankfurt/Main, Germany
Submission: August 21, 2017; Published: November 07, 2017

ISSN 2637-7632
Volume1 Issue1
Abstract
Early discovery of liver fibrosis and cirrhosis is becoming more relevant because of enhanced incidence of hepatocellular carcinoma. There a many underlying factors in developing liver fibrosis (i.e. viral hepatitis, steatohepatitis). Diagnosis of liver fibrosis is difficult; chronic liver failure and less distinct fibrosis stages can be underestimated, when laboratory routine parameters and native ultrasound of the liver are unsuspicious. Liver biopsy is a common element of diagnostic workup in hepatic cirrhosis, alongside clinical examination and abdominal ultrasound, and is the accepted diagnostic gold standard. But there is no unitary system of histological classification used to evaluate the degree of fibrosis, and individual systems are often validated only for individual disease entities. On the other hand liver biopsy is of less tolerance for patients. In the last years serological markers for detecting liver fibrosis were developed with different validity. Various imaging modalities have been proposed as methods for assessing liver fibrosis by liver stiffness measurement. They are sufficient to approve the suspicious of liver fibrosis and/or to uncover unknown chronic liver failure. Studies showed the clinical usefulness of acoustic radiation force impulse shear wave elasticity imaging (ARFI-SWEI) is efficient as a preventive screening method to uncover fibrosis. The ARFI-SWEI system is integrated in an ultrasound device has a good accuracy and high reproducibility. Therapy of liver fibrosis depends on underlying disease and degree of liver failure. When liver failure can be cured liver fibrosis can regress. Direct antifibrotic drugs are actually not available but in progress.
Keywords: Liver fibrosis; Liver failure; Liver elastography; Ultrasound; Biomarkers; Antifibrotic therapy
Introduction
The adult human liver typically weighs approximately 1.5kg. It is the largest internal organ and plays many pivotal roles in intermediary metabolism (i.e. metabolism and clearance of xenobiotics, disposal of bile, synthesis for major serum proteins (albumin, clotting factors)); in the normal state, the liver is maintained at a size which provides substantial overcapacity and has a remarkable ability to regenerate in response to functional parenchymal loss even after 70% of the parenchyma is lost [1]. Liver fibrosis (LF) is part of the structural and functional alterations of liver tissue in most chronic liver diseases. It is one of the main prognostic factors as the amount of fibrosis is correlated with the risk of developing liver cirrhosis (LC) [2]. LF resulting from chronic liver injury is a central pathologic healing process in progressive chronic liver disease (CLD). Non-alcoholic or alcoholic fatty liver disease ((N)AFLD) as well as chronic viral hepatitis B (HBV) and C (HCV) are leading CLD in the western world [3] and major cause of morbidity and mortality worldwide [4,5]. Chronic liver injury leads to initiation and perpetuation of inflammatory processes, which, by a cascade of inter-related processes and pathways, leads to deposition of extracellular matrix (ECM) proteins including collagen fibers (fibrous tissue) as a wound healing response. But this response causes tissue scarring. Fibrosis is a dynamic process of hepatic homeostasis mediated by several cellular mediators. In particular, hepatic stellate cells (HSC) have a central role in the pathogenesis of liver fibrosis and comprise 15% of liver cell mass. HSC are activated following liver injury from a relatively quiescent lipid and vitamin A-storing phenotype to a myofibroblastic phenotype, capable of proliferation, contraction and fibrogenesis. Other cell types, such as endogenous portal fibro- and myofibroblasts derived from liver parenchymal cells undergoing epithelial-mesenchymal transition are also suggested to contribute to the myofibroblast pool fibroblasts [6,7]. When liver injury and inflammation are persistent and progressive, liver cannot regenerate normally and causes LF up to LC [8].
Fibrosis has been considered potentially reversible with elimination by removal of causative agents, while the end-stage of the pathological process, cirrhosis, has been considered irreversible and is difficult to treat [6,7].
In recent years the early discovery of progressive liver failure, LF and LC is becoming more popular because of enhanced incidence of hepatocellular carcinoma (HCC); importantly, the incidence of HCC will continue to escalate as chronic hepatitis C (HCV) reaches its maturity and as nonalcoholic steatohepatitis (NASH) and obesity become more prevalent in the Western World [9]. HCC is the third leading cause of cancer-related death worldwide [10,11]. Nearly 95% of all HCC are based on LC with an incidence up to 6% per year [12].
Mortality analysis shows that CLD as cause of death range on place 5 in Germany and the risk to develop LC is up to 5% [8]. Data analysis from the U.S. Department of Health and Human Services revealed that CLD range on place 12 of 15 leading causes of death in 2013 [13].
Epidemiologic data from Germany estimates that nearly 4-5 million people suffer because of CLD with or without LF;(N) AFLD and the activated form, (N)ASH, as well as HBV/HCV and hemochromatosis are the main factors in over 90% of the cases. (N)AFLD/ (N)ASH, are growing problems for people of the industrialized countries. Overweight defined as body-massindex (bmi) of 25.0-29.9kg/m2 and obese with bmi ≥30.0kg/ m2 are caused by less sporting activity, excess of calories and/or alcohol and are the main reasons for developing fatty liver and/ or steatohepatitis with elevated risk of LC and HCC [14,15]. The overweight prevalence is 28.8% and obese ranges from 2.3-12%, mostly affecting females [15]. Nowadays (N)AFLD associated liver cirrhosis is the second leading cause for liver transplant in the USA and will overtake HCV in the next years [16].
Often patients do not know about their possible liver failure because typical symptoms like fatigue or jaundice are missing until the late stage of the disease [17]. The life expectancy and prognosis of patients with LC is significant lower with 1-year mortality of 40% [18]. To prevent LC the aim is to uncover early stages of a LF and corresponding CLD.
Diagnostic of Liver Fibrosis
To estimate the probability of LF comprehensive evaluation of underlying disease and patient history (including drug history) as well as body mass indices are necessary followed by ascertainment of laboratory parameters. These include especially the blood count, liver enzymes (aspartate-aminotransferase (AST), alanine-aminotransferase (ALT), cholinesterase (CHE), gammaglutamyltransferase (GGT), alkaline phosphatase (AP)) and blood clotting factors. In vague suspicion of CLD additional screening for HBV and HCV, hemochromatosis, autoimmune hepatitis including primary sclerosing (PSC) or primary biliary cholangitis (PBC), M. Wilson and lack of alpha-1-antitrypsin is recommended (Figure 1) [19].
Figure 1: Algorithm for diagnostic chronic liver failure.
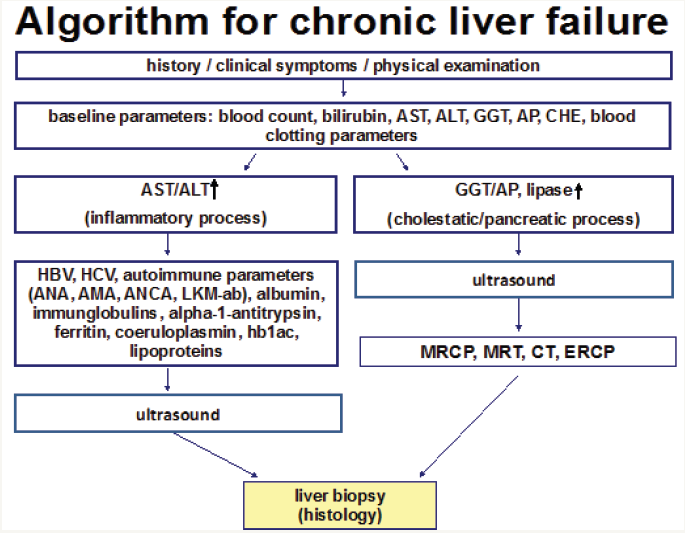
Invasive Diagnostic of Liver Fibrosis
In the past the diagnosis and follow-up of progressive LF by CLD based on histological examination using liver biopsy (LB) [20]. For patients with hepatitis of various etiologies, liver biopsy is used not only to establish the cause of the disorder, but also to assess the degree of inflammatory activity (grading) and the extent of fibrosis (staging). LB is an important aid to treatment planning and prognostication [21]. But recently its value as a method to assess the severity of liver disease (or to follow-up disease progression) has been questioned. The success of LB depends not only on the selection of the puncture method (i.e. percutaneous, ultrasoundguided liver biopsy (the Menghini method), trans jugular liver biopsy etc.) and on due attention to the relative and absolute contraindications, but also on the experience of the person carrying out the procedure. Although LB is still considered the “gold standard” for histological evaluation it is well known that this procedure has except poor tolerance as a stressful medical procedure for many patients several limitations [20,22]. Liver biopsy is expensive, it needs hospitalization for at least 6-18 hours, is invasive and carries a risk of complications with an associated morbidity rate between 0.3% and 0.6%, and with a mortality rate of 0.05% [23].
Liver biopsies only sample an extremely small portion of the liver (1/50,000) and therefore, sampling errors can occur, especially when smaller sized biopsies are analyzed [20,24,25]. Sampling mistakes and inter- and intra observer variations may result in under staging of cirrhosis or high graded fibrosis, which may occur even when widely validated systems are used to score liver damage (Table 1) [5]. The single histologic liver scores are established in context with special liver diseases and partially have different definitions for fibrosis stages.
Table 1: Different histopathologic staging systems.
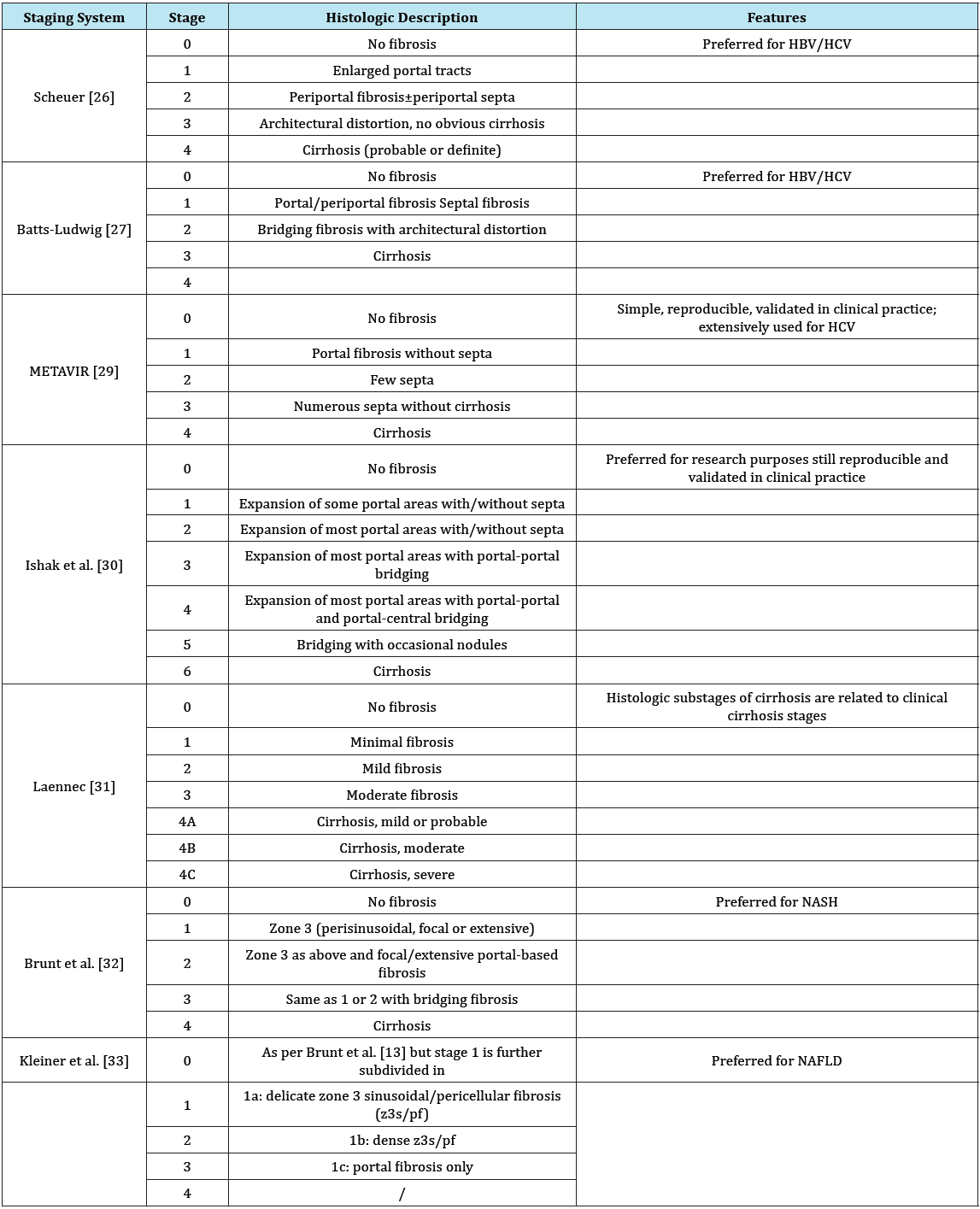
HBV: Chronic Hepatitis B; HCV: Chronic Hepatitis C; NAFLD: Nonalcoholic Fatty Liver Disease; NASH: Nonalcoholic Steatohepatitis [5].
Noninvasive Diagnostic of Liver Fibrosis
Thus, in recent years interest increased in identifying and describing liver fibrosis by using non invasive technical methods [20]. Noninvasive methods can be divided in two categories: serological and imaging-based technologies.
Serum Biomarkers
Serum biomarkers have been studied in detail to detect early fibrotic changes as blood tests are quick and acceptable to patients [6]. Today there is wide range of serum biomarkers used to identify liver damage. These biomarkers can be divided into two broad categories -direct and indirect. Indirect markers reflect liver function, which may decline with the onset of higher graded liver fibrosis or cirrhosis (i.e. blood clotting parameters, platelet count, cholinesterase). Direct markers reflect extracellular matrix (ECM) turnover, and include many molecules involved in hepatic fibrogenesis (i.e. hyaluronic acid, amino terminal of serum procollagen III peptide (PIIINP), tissue inhibitor of metalloproteinase (TIMP-1) [33]. In total there are a lot of indirect and direct test methods used in various contexts (i.e. HBV/HCV, NAFLD). The advantage of indirect test is using serological routine parameters. Direct tests are more expensive and often they are available to a limited extent in hospitals. Because of its quantity only few established tests are listed here (Table 2).
Table 2: Indirect and direct tests for liver fibrosis.
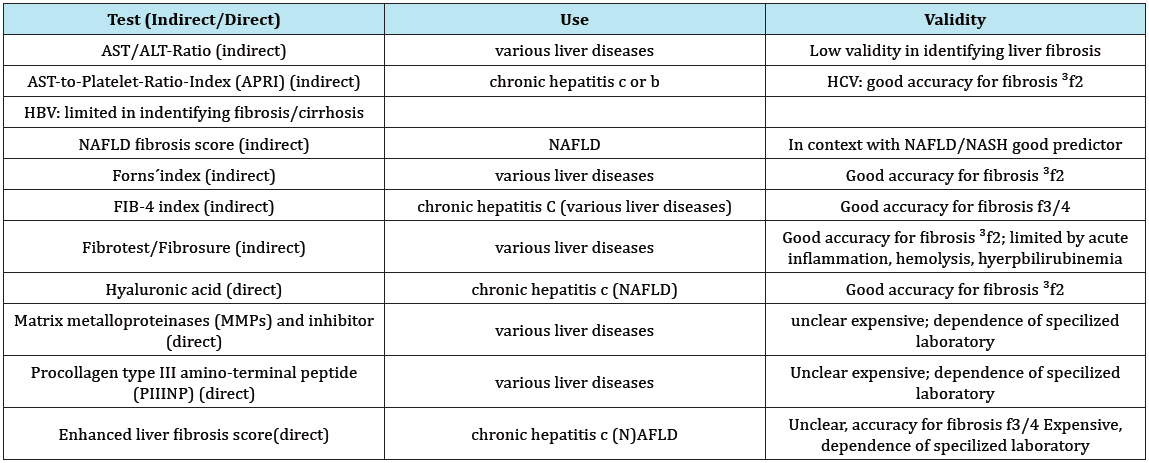
Indirect Tests
AST/ALT-ratio
The AST/ALT-ratio (AAR) or De-Ritis-Quotient used more than 30 years describes the relation of AST and ALT. It finds use in estimation of various liver diseases. The range of the dimensionless ratio is between 0.6-0.8. The area under the receiver operating characteristic curve (AUROC) for LF≥F3 varies between 0.68-0.78. In daily routine AST/ALT-ratio <1 reflects slightly and ratio >1 severe liver failure (i.e. cirrhosis) [34].
AST-to-platelet-ratio-index (APRI)
This index was developed by Wai et al. [35]. APRI was proposed as an alternative to biopsy in patients with chronic HCV infection and it is calculated as (AST/upper limit of normal range)/platelet count (109/L)×100. A large meta-analysis by Lin et al. suggest that APRI can identify HCV-related fibrosis with a moderate degree of accuracy and AUROC scores for the diagnosis of significant fibrosis (≥F2) between 0.77-0.83 respectively [36]. But APRI seems to be limited in identifying hepatitis B-related significant fibrosis and cirrhosis [37].
NAFLD Fibrosis score
Angulo et al. established a score including age, hyperglycemia, bmi, platelet count, albumin and AST/ALT-ratio as a good predictor (AUROC 0.88) for progressive liver fibrosis (F3-4) [38].
Forns index
Forns et al. developed a multivariate analysis-based model in a cohort of 476 subjects. The score includes four variables: age, GGT, cholesterol, platelet count. The usefulness of this index is restricted to patients with early-stage fibrosis (negative predictive value to exclude fibrosis≥F2 96% for scores below 4.2) [6,39].
FIB-4 index
This index combines the liver enzymes AST/ALT, the platelet count and age into the formula: age (years)×AST (U/L)/(platelets (109/L)×ALT (U/L)). The FIB-4 index was specifically developed as an alternative to biopsy in patients with chronic HCV infection, but has shown use in other causes of liver disease. In a study of 529 HCV-infected patients, the FIB-4 index enabled the correct identification of patients with severe fibrosis (F3-F4) and cirrhosis AUROC of 0.85 and 0.91, respectively [40].
Fibrotest®/Fibrosure®
Fibrotest® (Bio Predictive, Paris, France) and Fibrosure® (Lab Corp, Burlington, NC, USA) use five different serum markers: α2-macroglobulin, haptoglobin, apolipoprotein A1, GGT and total bilirubin. In contrast to the other indirect fibrosis tests, calculation of the Fibrotest/Fibrosure by a patented algorithm is subject to payment of a fee to the manufacturer. It has been validated in metaanalysis in multiple etiologies including NAFLD (AUROC 0.84), alcohol-related liver disease (AUROC 0.86) as well as chronic viral infection (HBV: AUROC 0.80; HCV: AUROC 0.85) [6,41]. Test accuracy is limited by acute inflammation, hemolysis or hyperbilirubinemia [42,43].
Direct fibrosis tests
Serum levels of ECM protein reflect the balance between hepatic fibrogenesis and fibrolysis and have been proposed as direct (bio) markers of hepatic fibrosis. Several fibrosis panels, combinations of such biomarkers, have been developed for commercial use. Their diagnostic performance in hepatic fibrosis may be limited by extrahepatic confounding factors such as systemic inflammation or renal failure [43].
Hyaluronic acid (HA)
HA is a polysaccharide present in ECM and elevated in serum in patients with hepatic fibrosis. The diagnostic accuracy was confirmed for fibrosis≥F2 in a large study of 486 HCV Patients (AUROC 93-99%) and in context with NAFLD [44,45].
Matrix metalloproteinases (MMPs) and inhibitor
Excess ECM proteins are degraded by MMPs which are in turn inhibited by tissue inhibitors of metallo proteinases (TIMPs). Both MMPs and TIMps are related to matrix protein turnover. But their usefulness is unclear because of conflicting results in context with LF in various studies [43].
Procollagen type III amino-terminal peptide (PIIINP)
In the healthy human liver the most abundant collagens are the fibril-forming types I and III. In its mature form, the collagen is integrated into the ECM, and its relative concentration in the basement membrane is higher in hepatic fibrogenesis followed by an increase in serum levels. In CLD, serum PIIINP reflects the stage of LF, but it is not specific for LF (i.e. also elevated in lung fibrosis, rheumatologic disease) [46].
Enhanced liver fibrosis score
The enhanced liver fibrosis score (ELF®) score (Siemens, Munich, Germany) is a combination of three direct markers of LF: hyaluronic acid, TIMP-1 and PIIINP. A higher score will indicate a higher rate of fibrogenesis. The test has good performance for detection significant fibrosis in chronic HCV (93% sensitivity and 83% specificity), in NAFLD (sensitivity 89% and specificity 96%) and ALD (100% sensitivity and 16.7% specificity). Test-results can be influenced by gender, age, and sex [6,47-49].
Imaging Based Methods
Standard ultrasound
Native ultrasound in b-mode for estimation liver tissue is important for screening liver damage or lesions. But its sensitivity for detection LF is low and higher graded LF can be underestimated.
Liver elastography, liver stiffness measurement
In the recent years various imaging modalities have been proposed as methods for assessing LF by liver stiffness measurement. Transient elastography (TE; FibroScan®, Echosens, Paris) was first developed. Briefly, this system is equipped with a probe consisting of an ultrasonic transducer mounted on the axis of a vibrator. A vibration of mild amplitude and low frequency is transmitted from the vibrator to the tissue by the transducer itself. This vibration induces an elastic shear wave which propagates through the tissue. In the meantime, pulse‐echo ultrasonic acquisitions are performed to follow the propagation of the shear wave and measure its velocity, which is directly related to tissue stiffness (or elastic modulus). The harder the tissue, the faster the shear wave propagates (AUROC:≥F3 0.93) [50].
Several studies substantiated good accuracy of Magnetic Resonance Elastography (MRE) for estimation LF. A prospective, cross-sectional study of more than 100 patients, demonstrated MRE to be more accurate than TE in identification of LF (≥F1) [51]. Studies utilize visual morphometry (MRI T1 sequence) to quantify the amount of fibrosis in liver biopsy and showed AUROCs for advanced fibrosis and cirrhosis of 0.931 and 1.000 respectively for pathologists versus 0.763 and 0.972 for T1 MRI sequence [52]. The advantage of this method is the evaluation of great volume of hepatic tissue and shows the often heterogeneous lesion distribution as well as it quantifies fibrosis, fat content, and iron content in the same 25min examination.
Acoustic Radiation Force Impulse Shear Wave Elasticity Imaging (ARFI-SWEI) is a novel ultrasound method for LF assessment [53- 55]. This dynamic method is a real time dual display-imaging mode and allows a quantitative assessment of tissue stiffness by using acoustic radiation force to produce an acoustic “push” pulse that generates shear waves, which propagate into the tissue; their speed (meter/second, m/s) reflects the underlying tissue stiffness and severity of LF. This method is comfortable for the patient and examiner and takes only few minutes. It can be used for assessing different degrees of liver stiffness (fibrosis stages) and correlated to the b-mode aspect of the liver in the same session [54,56].
In the last years studies verified ARFI-SWEI as a powerful noninvasive method in predicting fibrosis ≥F2 with high diagnostic accuracy and validity. The accuracy of ARFI-SWEI is well tested and it is reliable in prediction of fibrosis in early stages (≥F2). The sensitivity and specificity of the ARFI-SWEI method is ascertained of 83-88% and 89-90% and its accuracy, expressed as area under the curve receiver-operating characteristic (AUROC), is near 1 [56].
Many studies proofed the impact of possible influencing factors of ARFI measurements or SWV values, for example obese, small intercostal space, food intake, ultrasound transducer, operators expertise, cholestasis, breathing maneuvers or body position [57,58].
Recently a study on 382 patients with aim to investigate the clinical usefulness of ARFI-SWEI- screening for uncovering possible LF during routine ultrasound of the abdomen showed that in cases of normal native ultrasound scan liver damage can be underestimated. By using ARFI-SWEI as an additional method tissue damage of the liver was uncovered for further diagnostic evaluation [60].
Therapeutic options in liver fibrosis
If cirrhosis is not completed regression of fibrosis is possible, when underlying disease is treated. Recent clinical studies comprising patients successfully treated for viral hepatitis showed that liver fibrogenesis and even cirrhosis may be reverted [54]. Today successful therapeutic options for HBV and HCV minimize the risk of progressive LF up to LC and/or HCC. HCV treatment with direct acting antivirals is successful with sustained viral response over 92-98% and therapy of HBV with nucleotide-/nucleosideanalog- inhibitors are able to suppress viral load and initiate regression of fibrosis [60].
Until today there exists no direct anti-fibrotic drug for treating fibrosis. The hepatic capacity to remodel scar tissue and to revert into a normal liver follows specific mechanistic principles that include the termination of chronic tissue damage, shifting the cellular bias from inflammation to resolution, initiation of myofibroblast apoptosis or senescence and, finally, fibrinolysis of excess scar tissue. The plurality of molecular and cellular triggers involved in initiation, progression and resolution of hepatic fibrogenesis offers an infinite number of therapeutic possibilities [61]. For instance, inflammatory macrophages can be targeted via inhibition of chemokine or its receptor (i.e. by Cenicriviroc) as well as by transfer of restorative macrophage subsets [62]. Another target is galectin-3 that acts at various stages along the continuum from acute to chronic inflammation. Profibrogenic cytokines like transforming growth factor-β, matrix cellular proteins or signaling pathways involved in fibrogenesis offer further possible targets. Other options are the application of therapeutic antibodies directed against components involved in biogenesis or remodeling of connective tissue such as lysyl-oxidase-like-2 or synthetic bile acids like obeticholic acid that activate the farnesoid X receptor and was antifibrotic in a phase 2 study (FLINT trial) [63,64]. Obeticholic acid has already been proven to have efficacy when combined with ursodeoxycholic acid in the treatment of PBC [65]. Factors affecting the gut barrier function or the intestinal microbiome further expanded the repertoire of drug targets.
Animal studies showed positive side effects of the dipeptidyl peptidase-4 inhibitor (DPP4-I), Sitagliptin, attenuating LF via suppression of activated hepatic stellate cell and collagen synthesis in rats. Since DPP4-I is widely used in clinical practice, this drug may represent a potential new therapeutic strategy against LF in the future, and also in combination with angiotensin-II type 1 receptor blocker, Lorsatan [66,67].
Recently, Sorafenib, an FDA approved molecular targeted drug for the treatment of advanced hepatocellular and renal cell carcinomas, has been reported to exert anti-fibrotic effects in LF. Animal models showed that Sorafenib ameliorated intrahepatic vascular resistance, reduced portal hypertension, and reduced intrahepatic fibrosis, inflammation and angiogenesis. Further studies are required to clarify its anti-fibrotic role, effective dose, and side effects [68,69].
Conclusion
LF is part of the structural and functional alterations of liver tissue in most chronic liver diseases with the risk to develop LC. To avoid LB several non-invasive methods have been suggested for the diagnosis of LF, including serum markers, liver stiffness measurements and ultrasound parameters. Serum parameters are useful but complex scores including direct biomarkers are expensive or unavailable in daily use. Research results have shown their high diagnostic accuracy for advanced LF/LC. Native ultrasound gives hint for LF but can underestimate high graded liver fibrosis. TE and ARFI-SWEI may play a pivotal role in the study of LF. Studies have shown that elastography can detect both the progression and regression of fibrosis in individual course. To that fact the best validity seems to have ARFI-SWEI, because with this method LF≥F2 and liver failure can be uncovered. This easy handling method together with indirect fibrosis tests the accuracy in detecting liver fibrosis is higher. LF stops when underlying cause for liver failure is eliminated. The liver is capable to regenerate itself. Until today no drug for treating LF directly is available. However, it is still unknown if either non-invasive biomarkers of LF or elastography may contribute to a more accurate staging of LC, in terms of prognosis and fibrosis regression after effective therapy. In fact, not enough studies have shown both the fibrosis regression in different cirrhosis stages and the point beyond which the prognosis does not change - even in the event of fibrosis regression. Therefore, future studies are needed to validate non-invasive methods in predicting the different phases of liver LF.
Competing Interest
The author declare that he has no competing interests.
Financial Disclosure
The author has no financial disclosure to report and no conflict of interest.
References
- Wallace K, Burt AD, Wright MC (2008) Liver fibrosis. Biochem J 411(1): 1-18.
- European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higadp (2015) EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 63(1): 237-264.
- Schütte K, Kipper M, Kahl S, Bornschein J, Götze T, et al. (2013) Clinical characteristics and time trends in etiology of hepatocellular cancer in Germany. Digestion 87(3): 147-159.
- Altamirano BA, Beatriz BF, Nahum MS (2017) Management strategies for liver fibrosis. Ann Hepatol 16(1): 48-56.
- Almpanis Z, Demonakou M, Tiniakos D (2016) Evaluation of liver fibrosis: “Something old, something new...”. Ann Gastroenterol 29(4): 445-453.
- Karanjia RN, Crossey MM, Cox IJ, Fye HK, Njie R, et al. (2016) Hepatic steatosis and fibrosis: Non-invasive assessment. World J Gastroenterol 22(45): 9880-9897.
- Seki E, Brenner DA (2015) Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci 22(7): 512-528.
- Tacke F, Weiskirchen R (2010) Liver fibrosis - pathogenesis and novel therapeutic approaches. Internist (Berl) 51(1): 21-29.
- Balogh J, Victor D, Asham EH, Burroughs SG, Boktour M, et al. (2016) Hepatocellular carcinoma: a review. J Hepatocell Carcinoma 3: 41-53.
- Aghemo A, Colombo M (2013) Hepatocellular carcinoma in chronic hepatitis C: from bench to bedside. Semin Immunopathol 35(1): 111- 120.
- Paradis V (2013) Histopathology of hepatocellular carcinoma. Recent Results Cancer Res 190: 21-32.
- Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, et al. (2012) First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafe Nib) non-interventional study. Int J Clin Pract 66(7): 675-683.
- Xu J, Murphy SL, Kochanek KD, Bastian BA (2016) Deaths: Final Data for 2013. Natl Vital Stat Rep 64(2): 1-119.
- WHO (2000) Obesitiy: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Sci 894: 1-253.
- Poobalan A, Aucott L (2016) Obesity Among Young Adults in Developing Countries: A Systematic Overview. Curr Obes Rep 5(1): 2-13.
- Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, et al. (2015) Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148(3): 547-555.
- Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, et al. (2014) GIDEON (Global Investigation of therapeutic Decisions in hepatocellular carcinoma and of its treatment with sorafe Nib): second interim analysis. Int J Clin Pract 68(5): 609-617.
- Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, et al. (1987) Compensated cirrhosis: natural history and prognostic factors. Hepatology 7(1): 122-128.
- Bert F, Rindermann A, Abdelfattah MA, Stahmeyer JT, Rossol S, et al. (2016) High prevalence of chronic hepatitis B and C virus infection in a population of a German metropolitan area: a prospective survey including 10215 patients of an interdisciplinary emergency unit. Eur J Gastroenterol Hepatol 28(11): 1246-1252.
- Martínez SM, Crespo G, Navasa M, Forns X, et al. (2011) Noninvasive assessment of liver fibrosis. Hepatology 53(1): 325-335.
- Tannapfel A, Dienes HP, Lohse (2012) The indications for liver biopsy. Dtsch Arztebl Int 109(27-28): 477-483.
- Sporea I, Sirli R, Popescu A, Danilă M (2010) Acoustic Radiation Force Impulse (ARFI)--a new modality for the evaluation of liver fibrosis. Med Ultrason 12(1): 26-31.
- Abdollahi M, Pouri A, Ghojazadeh M, Estakhri R, Somi M (2015) Noninvasive serum fibrosis markers: A study in chronic hepatitis. Bioimpacts 5(1): 17-23.
- Bedossa P, Dargère D, Paradis V (2003) Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38(6): 1449-1457.
- Cadranel JF, Rufat P, Degos F (2000) Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32(3): 477-481.
- Scheuer PJ, (1991) Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 13(3): 372-374.
- Batts KP, Ludwig J (1995) Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 19(12): 1409-1417.
- Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, et al. (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38(2): 518- 526.
- Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24(2): 289-293.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, et al. (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22(6): 696-699.
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, et al. (2007) The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45(4): 846-854.
- Wanless IR, Sweeney G, Dhillon AP, Guido M, Piga A, et al. (2002) Lack of progressive hepatic fibrosis during long-term therapy with deferiprone in subjects with transfusion-dependent beta-thalassemia. Blood 100(5): 1566-1569.
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-TBA, Bacon BR (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94(9): 2467-2474.
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, et al. (2005) Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41(16): 1313-1321.
- Sharma S, Khalili K, Nguyen GC (2014) Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol 20(45): 16820- 16830.
- Williams AL, Hoofnagle JH (1988) Ratio of serum aspartate to alanine amino transferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 95(3): 734-739.
- Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, et al. (2011) Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 53(3): 726-736.
- Jin W, Lin Z, Xin Y, Jiang X, Dong Q, et al. (2012) Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol 12: 14.
- Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, et al. (2002) Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 36(4 Pt 1): 986-992.
- Taneja S, Tohra S, Duseja A, Dhiman RK, Chawla YK, et al. (2016) Noninvasive Assessment of Liver Fibrosis By Transient Elastography and FIB4/APRI for Prediction of Treatment Response in Chronic Hepatitis C-An Experience from a Tertiary Care Hospital. J Clin Exp Hepatol 6(4): 282-290.
- Poynard T, Morra R, Halfon P, Castera L, Ratziu V, et al. (2007) Metaanalyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 7: 40.
- Shaheen AA, Wan AF, Myers RP (2007) FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 102(11): 2589-2600.
- Stauber RE, Lackner C (2007) Noninvasive diagnosis of hepatic fibrosis in chronic hepatitis C. World J Gastroenterol 13(32): 4287-4294.
- McHutchison JG, Blatt LM, de Medina M, Craig JR, Conrad A, et al. (2000) Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol 15(8): 945-951.
- Suzuki A, Angulo P, Lymp J, Li D, Satomura S, et al. (2005) Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int 25(4): 779-786.
- Gressner OA, Weiskirchen R, Gressner AM (2007) Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liverdependent malfunction tests. Clin Chim Acta 381(2): 107-113.
- Rosenberg WM et al. (2004) European Liver Fibrosis Group. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 127(6): 1704-1713.
- Parkes J, Roderick P, Harris S, Day C, Mutimer D, et al. (2010) Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 59(9): 1245-1251.
- Pinzani M (2010) The ELF panel: a new crystal ball in hepatology? Gut 59(9): 1165-1167.
- Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, et al. (2006) Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 55(3): 403-408.
- Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, et al. (2017) Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 152(3): 598-607.
- Agrawal S, Hoad CL, Francis ST, Guha IN, Kaye P, et al. (2017) Visual morphometry and three non-invasive markers in the evaluation of liver fibrosis in chronic liver disease. Scand J Gastroenterol 52(1): 107-115.
- Gallotti A, D Onofrio M, Pozzi Mucelli R (2010) Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med 115(6): 889-897.
- Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, et al. (2011) Noninvasive evaluation of hepatic fibrosis using acoustic radiation forcebased shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol 55(3): 666-672.
- Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, et al. (2013) EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 34(2): 169-184.
- Friedrich-RM, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-BC, et al. (2012) Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat 19(2): e212-219.
- Goertz RS, Egger C, Neurath MF, Strobel D (2012) Impact of food intake, ultrasound transducer, breathing maneuvers and body position on acoustic radiation force impulse (ARFI) elastometry of the liver. Ultraschall in Med 33(4): 380-385.
- Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, et al. (2011) Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 54(4): 650-659.
- Bert F, Stahmeyer JT, Rossol S (2016) Ultrasound Elastography Used for Preventive Non-Invasive Screening in Early Detection of Liver Fibrosis. J Clin Med Res 8(9): 650-655.
- Grossi G, Viganò M, Loglio A, Lampertico P (2017) Hepatitis B virus longterm impact of antiviral therapy nucleot(s)ide analogues (NUCs). Liver Int 37(Suppl 1): 45-51.
- Yoon YJ, Friedman SL, Lee YA (2016) Antifibrotic Therapies: Where Are We Now? Semin Liver Dis 36(1): 87-98.
- Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, et al. (2016) Efficacy and safety study of cenicriviroc for the treatment of nonalcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials 47: 356-365.
- Makri E, Cholongitas E, Tziomalos K (2016) Emerging role of obeticholic acid in the management of nonalcoholic fatty liver disease. World J Gastroenterol 22(41): 9039-9043.
- Weiskirchen R, Tacke F (2016) Liver Fibrosis: From Pathogenesis to Novel Therapies. Dig Dis 34(4): 410-422.
- de Vries E, Beuers U (2017) Management of cholestatic disease in 2017. Liver Int 37(Suppl 1): 123-129.
- Okura Y, Namisaki T, Moriya K, Kitade M, Takeda K, et al. (2017) Combined treatment of dipeptidyl peptidase-4 inhibitor (sitagliptin) and angiotensin-II type 1 receptor blocker (losartan) suppresses progression in a non-diabetic rat model of steatohepatitis. Hepatol Res doi: 10.1111/hepr.12860.
- Kaji K, Yoshiji H, Ikenaka Y, Noguchi R, Aihara Y, et al. (2014) Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. J Gastroenterol 49(3): 481-491.
- Ma R, Chen J, Liang Y, Lin S, Zhu L, et al. (2017) Sorafenib: A potential therapeutic drug for hepatic fibrosis and its outcomes. Biomed Pharmacother 88: 459-468.
- Lin TsT, Gao DY, Liu YC, Sung YC, Wan D, et al. (2016) Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J Control Release 221: 62-70.
© 2017 Florian Bert. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






