- Submissions

Full Text
Global Journal of Endocrinological Metabolism
Sodium Glucose Cotransporter-2 Inhibitors for Gout Prophylaxis
Nasser Mikhail MD*
Department of Medicine, Endocrinology division, Olive-View-UCLA Medical Center, David- Geffen UCLA Medical School, USA
*Corresponding author: Nasser Mikhail MD*, Department of Medicine, Endocrinology division, Olive-View- UCLA Medical Center, David-Geffen UCLA Medical School, USA
Submission: January 06, 2023; Published: January 20, 2023

ISSN 2637-8019Volume3 Issue4
Abstract
Background: Studies have shown that use of Sodium-Glucose Cotransporter 2 (SGLT2) inhibitors
decreased serum uric acid concentrations.
Objective: To review the potential use of SGLT2 inhibitors for prevention of gout
Methods: Pub med search until January 5, 2023. Search terms included SGLT2 inhibitors, uric acid, gout,
treatment, diabetes. Randomized trials, meta-analysis, and guidelines are reviewed.
Results: SGLT2 inhibitors decreased serum uric acid levels by an average 0.55mg/dl (95% CI, 0.62 to
0.48, P<0.001) compared with placebo or comparator drugs. The magnitude of reduction in serum uric
acid with SGLT2 inhibitors was more pronounced with higher baseline serum uric acid levels and in
patients without type 2 diabetes. The decrease in serum uric acid concentrations occurred as early as 3
days after administration of SGLT2 inhibitor, was dose-independent, and was stable during the duration
of administration of SGLT2 inhibitors. Seven retrospective studies and 2 post-hoc analyses of large,
randomized trials showed that SGLT2 inhibitors reduced the risk of incident gout or the initiation of antigout
medications by 11-63%, whereas only one retrospective study did not report any significant effect.
Conclusion: SGLT2 inhibitors should be considered for gout prophylaxis in subjects with hyperuricemia.
Randomized trials are needed to define the optimum and cost-effective use of SGLT2 inhibitors for gout
prevention.
Keywords:SGLT2 inhibitors; Uric acid; Gout; Prevention; Type 2 diabetes; Heart failure
Introduction
SGLT-2 inhibitors, initially introduced as treatment for type 2 diabetes, were subsequently shown to decrease cardiac and renal events in patients with and without diabetes [1-3]. In addition, as a class effect, accumulating evidence suggested that use of SGLT2 inhibitors was associated with reduction in serum uric acid levels [4]. In fact, the decrease in circulating uric acid may contribute to the cardiac benefits of SGLT2 inhibitors [5]. High serum levels of uric acid are the most important risk factor for the development of gout [6]. Moreover, hyperuricemia may be an independent risk factor for coronary artery disease morbidity and mortality and for new-onset Chronic Kidney Disease (CKD) [7,8]. The main purpose of this article is to summarize data showing the impact of SGLT2 inhibitors on serum uric acid values and the incidence of gout.
Effects of SGLT2 Inhibitors on Serum Uric Acid Levels
In a recent meta-analysis of 43 randomized trials of SGLT2 inhibitors including 31,921 patients, Yip ASY et al. [4] found that SGLT2 inhibitors (empagliflozin, dapagliflozin, canagliflozin, luseogliflozin and ipragliflozin) significantly decreased uric acid concentrations compared with placebo or comparator anti-diabetic drugs by a mean of 0.55mg/dl (95% CI, 0.62 to 0.48, P<0.001). While significant reduction in mean serum uric acid levels was observed with all SGLT2 inhibitors (i.e. a class effect), luseogliflosin was associated with the greatest reduction (0.8mg/dl, 95% CI, 1.33 to 0.26), followed by canagliflozin 0.61 (95% CI, 0.71 to 0.51), empagliflozin 0.59mg/dl (95% CI, 0.71 to 0.47), then dapagliflozin had a mean reduction of 0.51mg/dl (95% CI, 0.61 to 0.41), and finally ipragliflozin 0.34mg/dl (0.49 to 0.19) [4]. However, data regarding these differences in uric acid reductions should be interpreted with caution due to lack of head-to-head comparison between various SGLT2 inhibitors. Importantly, the dose of SGLT2 inhibitors did not influence the magnitude of uric acid reduction [4].
Other findings reported in this meta-analysis showed that the mean decreases in uric acid levels were more pronounced in patients without diabetes versus those with diabetes, 1.5mg/dl (95% 2.12 to 0.94) and 0.52 (95% CI, 0.62 to 0.43), respectively [4]. This observation was confirmed in post-hoc analyses of 2 randomized large trials of SGLT2 inhibitors [9,10]. In the first analysis of EMPEROR-reduced trial, the adjusted mean reductions of serum uric acid levels by empagliflozin versus placebo were 0.99 and 1.25mg/dl in patients with and those without diabetes, respectively [9]. In the second analysis of DAPAHF trial, corresponding reductions by empagliflozin were 0.70 and 0.95mg/dl, respectively [10]. In addition, there is consistent evidence showing that reduction of serum uric acid levels is greater with higher baseline uric acid levels. Thus, Ferreira JP et al. [11] reported that empagliflozin placebo-adjusted reductions in serum uric acid levels were 0.56mg/dl and 0.30mg/dl among patients with baseline uric acid ≥7.0mg/dl, and <7.0mg/dl, respectively. Likewise, Doehner W et al. [9] recorded corresponding reductions of 1.75mg/dl and 0.54mg/dl in patients with heart failure (with and without diabetes) having baseline serum uric acid values ≥7.2mg/ dl and of ≤5.5mg/dl, respectively.
Time Course of Reduction in Serum Uric Acid by SGLT2 Inhibitors
The reduction of serum uric acid by SGLT2 inhibitors was rapid and occurred as early as 3 days after starting therapy [12]. In a larger trial (n=3,676), serum uric acid was significantly decreased 4 weeks after starting empagliflozin, and the magnitude of reduction remained stable throughout the treatment period that lasted 100 weeks [9].
Decrease Risk of Gout by SGLT2 Inhibitors
Available studies that examined the effects SGLT2 inhibitors on risk of incident gout are either retrospective cohort investigations (7 studies) or post-hoc analyses of randomized trials (3 studies) [9,10,13-19]. These studies are summarized in table 1 and discussed below. Using a US nationwide insurance database, Fralick M et al. [13] compared incidence of gout between patients with type 2 diabetes newly prescribed a SGLT2 inhibitor versus a GLP- 1 agonist. Mean duration of follow-up was 302 and 261 days in users of SGLT-2 inhibitors and GLP-1 agonists, respectively. After 1:1 propensity score matching (n=119,530 patients in each group), risk of gout was decreased by 36% in the group prescribed SGLT2 inhibitors compared with GLP-1 agonists, Hazard Ratio (HR) 0.64 (95% CI, 0.57 to 0.72) [13].
Moreover, Fralick M et al. [13] performed sensitivity analysis to compare incidence of gout in SGLT2 inhibitor users with propensity-score matched new dipeptidyl peptidase-4 (DPP-4) users (n=97,442 in each group). They found that the HR of gout associated with SGLT2 inhibitor use was 0.66 (95% CI, 0.58-0.75) [13]. In a retrospective cohort study from Taiwan, Chung MC et al. [14] compared the incidence of gout in 47,405 patients with type 2 diabetes using SGLT2 inhibitors versus propensity-score matched subjects receiving DPP-4 inhibitors. During a follow-up of approximately 2.5 years, the incidence of gout was 11% less among patients using SGLT2 inhibitors compared with DPP-4 inhibitors, adjusted HR 0.89 (95% CI, 0.82-0.96, P=0.004) [14]. Subgroup analysis revealed that the decreased risk of gout was more pronounced in patients younger than 65 years versus those older than 65-year-old [14]. Furthermore, no effect on decreasing risk of gout with use of SGLT2 inhibitors was demonstrated in the subgroup of patients with CKD, HR 1.01 (95% CI, 0.86 to 1.19) [14]. Gouda M et al. [15] compared rates of new prescriptions of antigout/antihyperuricemic drugs among 1,197 Japanese patients with type 2 diabetes with another group of 1,197 propensityscore matched subjects receiving other oral antidiabetic agents. These authors found that use of SGLT2 inhibitors was associated with 63% lower risk of new prescription of antigout medications compared with patients receiving other oral agents; Relative Risk (RR) 0.37 (95% 0.20 to 0.68) [15].
Using the Danish nationwide health registries, Lund LC et al. [16] compared the risk of gout in 11,047 pairs of users of SGLT-2 inhibitors and propensity-score matched users of GLP-1 agonists. Over 3 years of follow-up, the incidence of gout was 42% lower in users of SGLT2 inhibitors compared with those using GLP-1 agonists, HR 0.58 (95% CI, 0.44 to 0.75) [16]. In a large retrospective population-based study in Hong Kong of median follow-up of 5.59 years, Zhou J et al. [17] reported a 51% lower risk of gout among patients with type 2 diabetes receiving SGLT2 inhibitors compared with users of DPP-4 inhibitors matched by propensity scoring, HR 0.49 (95% CI, 0.42 to 0.58, P<0.0001). Banerjee M et al. [18] analyzed pooled data from 5 studies (3 observational and 2 posthoc analysis of randomized trials) to include 568,010 patients with type 2 diabetes. They showed that SGLT2 inhibitors were associated with 30% decrease in incident gout/gout flares, HR 0.70, 95% CI, 0.59 to 0.84, P<0.0001 [18].
Interestingly, consistent benefits were also observed in patients without baseline hyperuricemia (HR 0.65, 95% CI, 0.47 to 0.89, P<0.01) [18]. Results of post-hoc analyses of large, randomized trials were in accordance with those of retrospective studies. Ferreira JP et al. [11] performed a post hoc analysis of the EMPAREG OUTCOME trial of empagliflozin. During median follow-up of 2.6 years, among 6,607 patients not taking antigout medications at baseline, 5.2% and 3.6% had a gout episode or initiated anti-gout treatment in the empagliflozin and placebo, respectively, HR 0.67 (95% CI, 0.53 to 0.85, P=0.001) [11]. Doehner W et al. [9] obtained similar results in patients with heart failure with reduced ejection fraction. Thus, empagliflozin (10mg/d) reduced events of clinically relevant hyperuricemia (defined as acute gout, or initiation of antigout therapy) by 32% compared with placebo, HR 0.68 (95% CI, 0.52 to 0.89, P=0.004) [9]. Moreover, in another study of subjects with heart failure, the DAPA-HF trial, a uric acid-lowering agent was initiated in 4.4% (n=104) of patients randomized to placebo compared with 2.1% (n=51) of patients randomized to dapagliflozin (P<0.001) over a median follow-up of 18.2 months [10]. On the other hand, Subramanian A et al. [19] was the only group that did not find significant difference in gout incidence between 8,650 patients with type 2 diabetes using SGLT2 inhibitor compared with similar number of users of DPP-4 inhibitors matched by propensity score (Table 1).
Table 1:Effects of SGLT2 inhibitors on risk of gout.
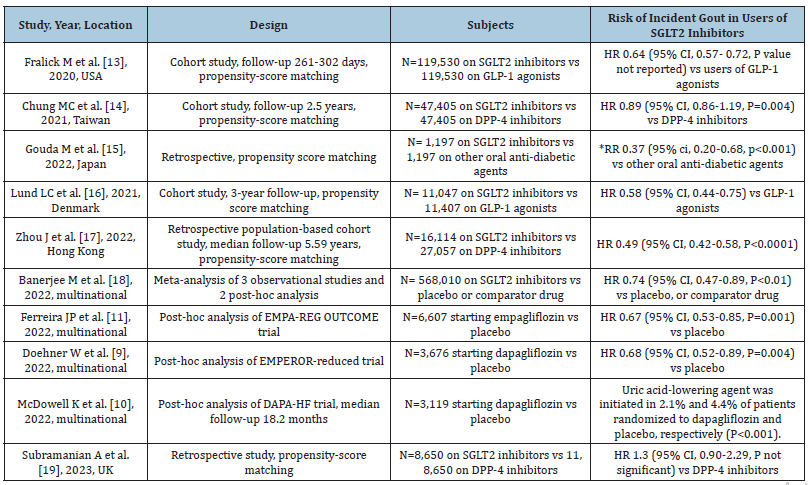
Abbreviations: SGLT2: Sodium-Glucose Co-Transporter-2; GLP-1: Glucagon-Like Peptide; DPP-4: Dipeptidyl Peptidase; HR: Hazard Ration; RR: Relative Risk of starting anti-gout medications
SGLT2 Inhibitors for Management of Hyperuricemia
There is only one published phase 2 clinical trial, the QUARTZ study, that compared the effect of dapagliflozin with placebo on urine and serum uric acid levels in subjects with hyperuricemia (serum uric acid >6.0mg/dl) [12]. All enrolled individuals (n=36) received 2 other anti-gout medications: verinurad, a urate transporter 1 inhibitor and febuxostat, a xanthine oxidase inhibitor [12]. The QUARTZ study included 7 day-treatment period of dapagliflozin therapy (10mg/day) and another 7 day-period of placebo separated by 7-21 days wash-out period in a cross-over design [12]. After 7 days of intervention, reduction in serum uric acid concentrations was significantly greater in the dapagliflozin group than placebo, placebo-adjusted difference 1.04mg/dl (95% CI, 1.39 to 0.70) [12]. Fractional excretion of uric acid in urine was 94% higher with dapagliflozin compared with placebo. Meanwhile, 24h uric acid excretion was decreased to a similar extent from baseline to day 7 in the dapagliflozin and placebo group [12]. These preliminary results suggest SGLT2 inhibitors may lower serum uric acid concentrations beyond the reduction achieved by other antigout medications. In the meantime, the discrepant finding showing that dapagliflozin did not increase the 24h urine excretion but increased fractional uric acid excretion requires further studies.
Mechanisms of Uric Acid Reduction by SGLT2 Inhibitors
The exact mechanisms whereby SGLT2 inhibitors lower serum uric acid levels are still unclear but are likely multifactorial. The most accepted mechanism is by increasing uric acid urinary excretion secondary to glycosuria [10,20]. There is also speculation that SGLT2 inhibitors might modulate the activity of urate transporters in the kidney tubules [10].
Rationale for Using SGLT2 Inhibitors for Gout Prophylaxis
There are 2 major reasons to consider using SGLT2 inhibitors as preventive drugs of gout in high-risk subjects, i.e., those with hyperuricemia. First, although available data are either retrospective or based on post-hoc analysis of randomized trials, they are consistent in a wide range of pathologies including type 2 diabetes, heart failure and CKD [9,10,13-19]. While the magnitude of reduction in serum uric acid levels may be modest, the decrease in the incidence of gout is much more pronounced and clinically significant. Second, gout is frequently associated with heart failure, type 2 diabetes, obesity and CKD [6]. In fact, cardiovascular disease is the main cause of increased mortality in gout [6,21]. Therefore, in view of the benefits of SGLT2 inhibitors in treatment of type 2 diabetes, induction of mild weight loss, and reduction of cardiorenal events, the use of SGLT2 inhibitors for gout prophylaxis becomes more justified in presence of any of these conditions. Currently, the use of urate-lowering drugs for the primary purpose of preventing incident gout is still controversial. Thus, only 4 of 24 guidance documents recommend treatment of asymptomatic hyperuricemia with serum uric acid cut-offs levels ranging from 8.0 to 13.0mg/ dl [22]. However, these recommendations were published in 2019 before results of studies of gout-prevention by SGLT2 inhibitors became available in 2020-2022 (Table 1). It is likely therefore that guidelines regarding gout prophylaxis might change in the light of these recent investigations.
Conclusion and Current Needs
Available data derived from cohort studies and post-hoc analysis of large, randomized trials worldwide showed that use of SGLT-2 inhibitors was associated with significant reduction in serum uric acid levels and 11-63% decreased risk of incident gout or initiation of antigout medications. The magnitude of reduction in serum uric acid levels is greater with higher baseline serum uric acid levels and among subjects without diabetes. Use of SGLT2 inhibitors for gout prophylaxis should be considered in predisposed individuals, i.e., those with hyperuricemia. Randomized trials are needed to help define which subgroups of subjects (in terms of baseline uric acid cutoff values, types of co-morbidities, age and gender) are likely to obtain the greatest risk reduction of gout to allow optimum and cost-effective use of SGLT2 inhibitors in this setting. Preliminary data suggest that SGLT2 inhibitors may decrease the degree of hyperuricemia on top of traditional anti-gout medications [12]. Therefore, it will be equally interesting to evaluate SGLT2 inhibitors as adjunctive therapy in patients with gout for prevention of recurrence of gout attacks.
Conflict of Interest
The author has no conflict of interest to declare.
References
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, et al. (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381(21): 1995-2008.
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, et al. (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383(15): 1413-1424.
- Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, et al. (2019) SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 7(11): 845-854.
- Yip ASY, Leong S, Teo YH, Teo YN, Syn NLX, et al. (2022) Effect of Sodium-Glucose Cotransporter-2 (SGLT2) inhibitors on serum urate levels in patients with and without diabetes: A systematic review and meta-regression of 43 randomized controlled trials. Ther Adv Chronic Dis 13: 20406223221083509.
- Fitchett D, Inzucchi SE, Zinman B, Wanner C, Schumacher M, et al. (2021) Mediators of the improvement in heart failure outcomes with empagliflozin in the EMPA-REG outcome trial. ESC Heart Fail 8(6): 4517-4527.
- Dalbeth N, Gosling AL, Gaffo A, Abhishek A (2021) Gout. Lancet 397(10287): 1843-1855.
- Li M, Hu X, Fan Y, Li K, Zhang X, et al. (2016) Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep 6: 19520.
- Li L, Yang C, Zhao Y, Zeng X, Liu F, et al. (2014) Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol 15: 122.
- Doehner W, Anker SD, Butler J, Zannad F, Filippatos G, et al. (2022) Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction: The EMPEROR-reduced trial. Eur Heart J 43(36): 3435-3446.
- McDowell K, Welsh P, Docherty KF, Morrow DA, Jhund PS, et al. (2022) Dapagliflozin reduces uric acid concentration, an independent predictor of adverse outcomes in DAPA-HF. Eur J Heart Fail 24(6): 1066-1076.
- Ferreira JP, Inzucchi SE, Mattheus M, Meinicke T, Steubl D, et al. (2022) Empagliflozin and uric acid metabolism in diabetes: A post hoc analysis of the EMPA-REG outcome trial. Diabetes Obes Metab 24(1): 135-141.
- Stack AG, Han D, Goldwater R, Johansson S, Dronamraju N, et al. (2021) Dapagliflozin added to verinurad plus febuxostat further reduces serum uric acid in hyperuricemia: The QUARTZ study. J Clin Endocrinol Metab 106(5): e2347-e2356.
- Fralick M, Chen SK, Patorno E, Kim SC (2020) Assessing the risk for gout with sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes: A population-based cohort study. Ann Intern Med 172(3): 186-194.
- Chung MC, Hung PH, Hsiao PJ, Wu LY, Chang CH, et al. (2021) Association of sodium-glucose transport protein 2 inhibitor use for type 2 diabetes and incidence of gout in Taiwan. JAMA Netw Open 4(11): e2135353.
- Gouda M, Arakawa K, Inagaki M, Ushirogawa Y (2022) Effect of sodium-glucose cotransporter 2 inhibitor medication on new prescriptions of antihypertensives, antigout/antihyperuricemics and Antidyslipidemics in Japan: Analysis using the JMDC Claims Database. J Diabetes Investig 13(11): 1842-1851.
- Lund LC, Højlund M, Henriksen DP, Hallas J, Kristensen KB (2021) Sodium-glucose cotransporter-2 inhibitors and the risk of gout: A Danish population based cohort study and symmetry analysis. Pharmacoepidemiol Drug Saf 30(10): 1391-1395.
- Zhou J, Liu X, Chou OH, Li L, Lee S, et al. (2022) Lower risk of gout in Sodium Glucose Cotransporter 2 (SGLT2) inhibitors versus Dipeptidyl Peptidase-4 (DPP4) inhibitors in type-2 diabetes. Rheumatology (Oxford)
- Banerjee M, Pal R, Mukhopadhyay S (2022) Can SGLT2 inhibitors prevent incident gout? A systematic review and meta-analysis. Acta Diabetol 59(6): 783-791.
- Subramanian A, Gokhale K, Sainsbury C, Nirantharakumar K, Toulis KA (2023) Sodium-glucose cotransporter-2 inhibitors and the risk of gout in patients with type 2 diabetes mellitus: A propensity-score-matched, new-user design study with an active comparator using the IQVIA medical research data UK database. Diabetes Obes Metab 25(1): 156-165.
- Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, et al. (2014) SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 35(7): 391-404.
- Clarson LE, Chandratre P, Hider SL, Belcher J, Heneghan C, et al. (2015) Increased cardiovascular mortality associated with gout: A systematic review and meta-analysis. Eur J Prev Cardiol 22(3): 335-343.
- Li Q, Li X, Wang J, Liu H, Kwong JS, et al. (2019) Diagnosis and treatment for hyperuricemia and gout: A systematic review of clinical practice guidelines and consensus statements. BMJ Open 9(8): e026677.
© 2023 Nasser Mikhail MD. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






