- Submissions

Full Text
Global Journal of Endocrinological Metabolism
Non-Enzymatic Glycation Protein in Type 1 Diabetes Mellitus: Diagnostic Value of Srage for Determining Metabolic Compensation and Early Detection of Vascular Risk in Childhood
Taranushenko TE1*, Kiseleva NG1, Lopatina OL1, Salmina AB1,2 and Salmin VV1
1Department of Pediatrics of Institute of Postgraduate Education, Russia
2Department of Neurology, Russian
*Corresponding author: Rangelova L, Department of National Center of Public Health and Analyses, Bulgaria
Submission: May 11, 2022; Published: June 16, 2022

ISSN 2637-8019Volume3 Issue4
Summary
To establish the diagnostic significance of the concentration of Soluble Receptors of Protein Glycation End Products (sRAGE) in children with type 1 diabetes mellitus.
Keywords: Diabetes mellitus; Children; Adolescents; Metabolic compensation; Non-enzymatic glycation; Soluble receptor for advanced glycation end products.
Backgraund
Type 1 Diabetes Mellitus (DM) is one of the most common chronic metabolic diseases [1]. According to the International Diabetes Federation, the average annual increase in morbidity in most European countries is about 3.4%. Considerable attention is currently being paid to the study of specific vascular pathology in DM, pathogenetic mechanisms, the nature of cellular damage, early markers of occurrence and progression.
Materials and Methods
Conducted a cross-sectional comparative descriptive study, determined by ELISA and analyzed the performance of plasma Soluble Receptor of Advanced Glycation End Products of Proteins (sRAGE) in patients with type 1 diabetes of both sexes from 6 to 17 years with disease duration of 1-10 years, studied correlation of sRAGE and levels of glycated hemoglobin with the use of multivariate regression analysis in the group of patients with diabetes [2].
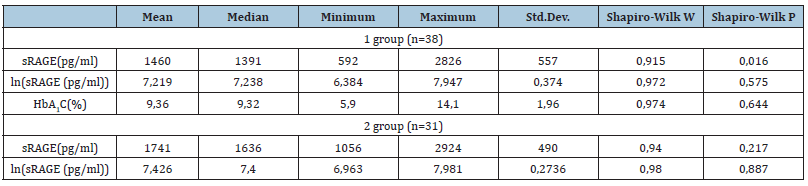
Result
The content of sRAGE in the blood was determined in 69 patients, of which 38 patients with type 1 diabetes (group 1), 31 were children of the first and second health groups without carbohydrate metabolism disorders (group 2). In patients with diabetes, it was mandatory to study the level of glycated hemoglobin (HbA1c) [3]. The indicators of plasma Soluble Receptors of Protein Glycation End Products (sRAGE) in the study groups were analyzed. Since the concentration of sRAGE in group 1 patients did not have a normal distribution on a uniform scale, this parameter was considered on the logarithmic ln(sRAGE(pg/ml) scale (Table 1). The results of the study revealed a significant decrease in the concentration of sRAGE in the plasma of patients with diabetes mellitus (p<0.05) [4]. Thus, the average, maximum and minimum sRAGE levels in group 1 were 1460, 2826 and 592pg/ml against similar indicators in group 2-1741, 2924 and 1056pg/ml, respectively (Table 1). The median sRAGE values in group 1 patients corresponded to 1391pg/ml, in children 2 groups-1636pg/ml. Analysis of the content of glycated hemoglobin in the serum of patients with DM showed unsatisfactory compensation of carbohydrate metabolism [5]. The average level of HbA1c in the study group was 9.36% with a minimum value of 5.9%, a maximum of 14.1% (median-9.32). It is likely that the decrease in the concentration of sRAGE in patients with DM is due to an increase in RAGE due to hyperglycemia, as well as a violation of the cleavage of sRAGE from RAGE, which may be mediated by endogenous insulin deficiency and secondary insulin resistance [6]. The data obtained suggest that low sRAGE values in patients with DM mark the degree of activation of the RAGE system and reflect the individual risk of vascular pathology [7]. The correlation between the level of sRAGE and glycated hemoglobin was studied using multivariate regression analysis in a group of patients with diabetes mellitus [8]. The results of the study revealed the dependence of the sRAGE indicator on the combined influence of several factors [9]. A significant regression model was found predicting the concentration of sRAGE by the level of glycated hemoglobin and puberty [10]. There were no gender differences with the changed sRAGE values. The presented data reflect the peculiarities of the influence of puberty on the metabolic compensation of diabetes [11]. It is known that the period of puberty is normally characterized by physiological insulin resistance and an increase in the secretion of counterinsular hormones [12]. These circumstances make it difficult to achieve the target values of glycemia in adolescents with diabetes [13]. The obtained data of the performed analysis established a significant effect on the concentration of sRAGE of a combination of elevated HbA1c and adolescence and allow us to attribute the puberty period to special risk factors in the formation of negative “metabolic memory” and in the occurrence of diabetic microangiopathies [14]. To study the relationship between sRAGE and HbA1c indicators in children with diabetes mellitus, an inverse regression model was created [15]. The results showed an inverse correlation and allowed the calculated level of HbA1c to be indicated by the concentration of sRAGE, gender and age [16]. The presented data reflect the diagnostic significance of soluble receptors of protein glycation end products in the assessment of metabolic memory and allow us to consider sRAGE as a predictor of the formation of glucose toxicity in children with type 1 diabetes mellitus. Discussion: In this paper, the indicators of plasma soluble receptors of protein glycation end products (sRAGE) in children with diabetes mellitus are analyzed [17]. A significant decrease in the plasma concentration of sRAGE was found in patients with the pathology under consideration (p<0.05), which is probably due to an increase in the expression of Receptors for Protein Glycation end Products (RAGE) due to hyperglycemia, as well as a violation of the cleavage of sRAGE from RAGE, mediated by endogenous insulin deficiency and secondary insulin resistance [18]. In a group of patients with diabetes mellitus, the correlation between the level of sRAGE and glycated hemoglobin was studied using multivariate regression analysis [19]. The dependence of the sRAGE indicator on the combined influence of several factors is revealed [20]. The combination of elevated HbA1c and adolescence has been found to have the most significant effect on the sRAGE concentration, which makes it possible to attribute puberty to modifiable risk factors in the formation of negative metabolic memory and in the occurrence of diabetic microangiopathies [21]. To study the relationship between sRAGE and HbA1c indicators in children with diabetes mellitus, an inverse regression correlation model was created that allows determining the calculated level of HbA1c by sRAGE concentration, gender and age [22]. The presented data reflect the diagnostic significance of soluble receptors of protein glycation end products in the assessment of metabolic memory [23]. Thus, the performed study made it possible to assess the level of plasma sRAGE in type 1 diabetes mellitus, to present an analysis of the functional relationship between the values of HbA1c, sRAGE, gender and age-sex factors, to clarify the diagnostic significance of sRAGE for determining metabolic compensation and early detection of the risk of vascular pathology in childhood [24]. The authors declare the absence of obvious and potential conflicts of interest related to the publication of this article [25].
Table 1:sRAGE values in the normal and logarithmic scale and the Shapiro-Wilk normality criterion in the examined patients.
References
- (2019) International Diabetes Federation (IDF), Diabetes Atlas, 9th (edn), Belgium.
- Chimikov AA, Severina AS, Shamhalova MS, ShestaKova MV (2017) The role of mechanisms of "metabolic memory" in the development and progression of vascular complications of diabetes mellitus. Diabetes Mellitus 20(2): 126-134.
- Dedov II, Shestakova MV ( 2015) The metabolic memory phenomenon in predicting the risk of vascular complications in diabetes mellitus. Ther Arkh 87(10): 4-10.
- Chernikov AA, Severina AS, Shamkhalova M, Shestakova MV (2017) The role of mechanisms of "metabolic memory" in the development and progression of vascular complications of diabetes mellitus. Diabetes Mellitus 20(2): 126-134.
- Titov VN, Khokhlova NV, Shiryaeva, Lu Ks (2013) Glucose, glycotoxins and protein glycation products: role in pathogenesis. Klin Med 3: 15-24.
- Leonova TS, Vihnina MV, Grishina TV, Leonova LE, Frolov AA, et al. (2018) Influence of deep glycation end products on cellular processes. International Research Journal 12(78).
- Ivannikova EV, Melkozerov KV, Kalashnikov V Yu, Terekhin SA, Kononenko IV, et al. (2013) Study of the role of fibroblast growth factors (bFGF, Tgfß1), inflammatory markers (IL-6, TNF-α, CRP) and glycation end products (AGE, RAGE) in patients with coronary heart disease and type 2. Diabetes Mellitus 16(3): 64-70.
- Bakunina NS, Glushakov RI, Tapilskaya NI, Shabanov PD (2013) Pharmacology of polyprenols as adaptogens that reduce the intensity of glycation processes. Reviews of Clinical Pharmacology and Drug Therapy 11(4): 44-53.
- Skobeleva KV, Tyrtova LV, Nikitina IL, Olenev AS (2019) Modern view on the problem of diabetic nephropathy in children and adolescents with type 1 diabetes: the role of renin-angiotensin-aldosterone systems. Attending physician 3.
- Zakharina OA, Tarasov AA, Babayev AR (2012) Current aspects of drug prevention and treatment of diabetic angiopathy. Medicinal Bulletin 5(45): 14-22.
- Ahmed N, Thornalley PJ (2009) The Role of glycation end products in the pathogenesis of diabetes complications. Russian Medical Journal 9: 642-650.
- Ansari NA, Rashid Z (2010) Non-enzymatic protein glycation: from diabetes to cancer. Biomed Khim 56(2): 168-178.
- Assumption A, Gorina YV, Salmin VV, Kovacheva N, Fursov AA, et al. (2014) The receptors of advanced glycation end products. Clinical Medicine 12(4): 68-76.
- Redkin Yu, Bogomolov VV, Dreval AV (2011) Influence of various factors on the effectiveness of self-control in diabetes. Consilium Medicum 12(13): 88-92.
- Ametov AS, Chernikova NA, Pugovkina Ya V (2016) Glucose homeostasis in a healthy person in various conditions. Modern View Endocrinology 1: 45-55.
- Yamagishi S, Nakamura N, Suematsu M (2015) Advanced glycation end products: a molecular target for vascular complications in diabetes. Mol Med 21(1): 32-40.
- Uspenskaya Yu, Komleva Yu K, Olenkova EA, Salmin VV, Lopatina OL, et al. (2015) Ligands of RAGE-proteins: role in intercellular communication and pathogenesis of inflammation. Vestn Ross Akad Med Nauk 70(6): 694-703.
- Nedosugova LV (2016) Possibilities of drug correction of diabetic polyneuropathy. Effective Pharmacotherapy 4: 46-52.
- Miao WD, Ming Li, Jie Chen, Tong-Tong Fu, Ke-Zhi Lin, et al. (2016) Activation of α7nachr promotes diabetic wound healing by suppressing age-induced TNF-α Inflammation 39(2): 687-699.
- Higashida H, Furuhara K, Yamauchi AM, Deguchi K, Harashima A, et al. (2017) Intestinal transepithelial permeability of oxytocin into the blood is dependent on the receptor for advanced glycation end products in mice. Scientific Reports 7(1): 1-15.
- Chinedum E, Norsuhana O, Oon Zhi L, Boon SY, Nik Hazlina, et al. (2019) Obesity and Comorbidity: could simultaneous targeting of esrage and srage Be the panacea? Front Physiol 10: 787.
- Peterkova VA (2016) Pediatric and adolescent diabetes: ISPAD clinical practice consensus guidelines September 2014.
- Dedov II, Shestakova MV, Mayorov A Yu (2019) Standards of specialized diabetes care 9th Diabetes Mellitus 22(1): 1-145.
- Dedova II, Peterkova VA, Kuraeva TL (2013) Diabetes mellitus in children and adolescents.
- Dedov II, Peterkova VA (2014) National clinical guidelines on endocrine diseases cure in children.
© 2022 Taranushenko TE. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






