- Submissions

Full Text
Global Journal of Endocrinological Metabolism
Serum Crosslaps (CTX) and 25hydroxyvitamin D Levels as Risk Predictors of Bisphosphonate- Related Osteonecrosis of the Jaw
Silva AMAPN1, Saldanha ALR1, Margeotto APP1, Silva LCPN1, Gasparoto ALV2, Martinez TLR1*
1Oral and Maxillofacial Surgery Department II, School of Dentistry, University of Buenos Aires, Department of Dentistry, Favaloro Foundation University Hospital, Argentina
2Oral and Maxillofacial Surgery Department II, School of Dentistry, University of Buenos Aires, Argentina
3,4Metabolic Bone Diseases Laboratory, Institute of Immunology, Genetics, and Metabolism (INIGEM), School of Pharmacy and Biochemistry, San José de San Martin Clinical Hospital (CONICET-UBA) Buenos Aires, Argentina
5President of the National Academy of Dentistry, Consultant to the National Academy of Medicine, Former Professor of Oral and Maxillofacial Surgery School of Dentistry University of Buenos Aires, Argentina
*Corresponding author: PhD Rey EA, President of the National Academy of Dentistry, Consultant to the National Academy of Medicine, Former Professor of Oral and Maxillofacial Surgery School of Dentistry University of Buenos Aires, Argentina
Submission: November 11, 2020; Published: February 04, 2021

ISSN 2637-8019Volume3 Issue3
Summary
Bisphosphonates (BPs) are anticatabolic drugs of choice for treating bone diseases, including bone metastases. Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is one of the possible complications. Crosslaps (CTX) could be used as biochemical marker for risk of developing ONJ. Vitamin D (VD) may be involved in this condition. VD status and CTX levels were evaluated and compared in BPtreated women without BRONJ (Group I; n=28) and with BRONJ (Group II; n=58). Women were older and duration of BP use was longer in Group II (p=0.0000036). No differences in calcemia, phosphatemia, or CTX levels were observed; BAP levels were significantly higher and 25OHD were significantly lower in Group II (p=0.040 and p=0.035, respectively). The percentage of subjects with CTX levels between 100 and 149mg/mL was similar in both groups. VD deficiency was observed in 18% of subjects in Group II but in none of the subjects in Group I. No significant differences in the percentage of subjects with VD insufficiency and sufficiency were observed between groups (Group I: 50%; Group II: 40%). Conclusion: CTX levels did not prove useful as predictors of risk for developing BRONJ. The high percentage of women with VD deficiency who developed BRONJ suggests a possible relationship between both conditions and highlights the importance of assessing Vitamin D status.
Keywords: Bisphosphonates; Osteonecrosis of the jaw; Women; Vitamin D; CTX
What are Glucocorticoids?
Bisphosphonates (BPs) are the anti-catabolic treatment of choice for osteoporosis and
other bone diseases [1]. Therapeutic response to BPs is affected by Vitamin D (VD) status,
which is determined by 25hydroxyvitamin D (25OHD) levels [2].
Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is one of the possible
complications of chronic oral or intravenous treatment with aminoBPs [3]. In 2007, the
American Association of Oral and Maxillofacial Surgeons (AAOMS) defined this side effect of
BP treatment as an area of exposed bone in the maxillofacial region in a patient on BP therapy
for more than eight weeks and who has not had radiation therapy to the head and neck region
[4]. Although the etiology of BRONJ is multifactorial, the marked decrease in bone remodeling
would be one of the main risk factors for its development [5].
Bone remodeling involves two highly coordinated events, namely bone formation due to
osteoblastic activity and bone resorption caused by osteoclastic activity. Bone cell activity
can be assessed using biochemical markers of bone formation and resorption. These markers
are used routinely to evaluate response to antiresorptive treatment. Through their action
on the mevalonate pathway, BPs block osteoclast activity and favor cell apoptosis inhibiting
bone resorption. Bone remodeling starts with a resorption event, so that inhibition of bone
resorption dramatically decreases bone tissue remodeling [6].
C-terminal telopeptide of collagen type I (CTX or Crosslaps)
is currently considered the most sensitive and specific marker of
bone resorption to assess changes in bone remodeling. Serum CTX
levels decrease markedly within the first weeks or month after
initiating anti catabolic treatment [7]. A new role for CTX has been
suggested over the last years. According to Marx RE et al., very low
serum CTX levels in patients on chronic BP therapy would indicate
potential risk for developing BRONJ [8]. Other authors, however,
found no relation between CTX levels and development of BRONJ
[9,10] and the clinical utility of CTX as a predictor of BRONJ remains
controversial to date [11-14].
Bedogni A et al. [15] found that 77% of patients with BRONJ
but only 5% of BP-treated patients who did not develop BRONJ had
osteomalacia [15]. Inadequate Vitamin D status has therefore been
proposed as an additional risk factor for ONJ in patients on longterm
treatment with bisphosphonates.
Based on the above, the present study sought to evaluate and
compare the VD status and CTX levels of women on chronic BP
therapy with and without BRONJ.
Materials and Methods
Subjects
The subjects were recruited from a population of 25.538
male and female patients, mean age 55±12 treated at the Oral
and Maxillofacial Traumatology and Surgery Department II of the
School of Dentistry, University of Buenos Aires (FOUBA), between
2007 and 2013. The present study included all the female patients
aged more than 20 years referred for oral surgery by their attending
dentist or physician during the study period. All subjects signed a
written informed consent form prior to enrollment.
The present study was conducted in keeping with ANMAT
5330/97 guidelines and regulations, and in compliance with
the Helsinki declaration and UNESCO bioethics principles. The
study was approved by the Ethics Committee of the institution
(Resolution (CD) 399).
Methods
From a total 253 women on long-term treatment with BPs
(mean age: 62.4±6.7 years) referred for oral surgery to the
department within the study period, 86 complied with the study
inclusion criteria and were assigned to one of two groups according
to the presence of BRONJ.
Group I (n=28): women receiving BPs who did not develop
BRONJ.
Group II (n=58): women receiving BPs who developed BRONJ.
Clinical diagnosis of BRONJ was made according to the 2014
update of the American Association of Oral and Maxillofacial
Surgery position papers on medication-related osteonecrosis
of the jaw [3]. Based on these guidelines, patients with a history
of radiation therapy to the head and neck, presenting a systemic disease that alters normal physiology of bone tissue, and/or
who did not consent to participate in the study or to undergo the
biochemical assessments of bone and phospho-calcium metabolism
were excluded from the study. BRONJ diagnosis was confirmed by
clinical assessment.
At the first consultation of each participant, a patient interview
was conducted and oral examination was performed to assess
clinical signs and symptoms, neural signs and symptoms, and
presence of oral lesions. The patient’s medication, underlying
disease (osteoporosis, Paget’s disease, cancer) prompting BP
therapy, and type of BP and duration of use regardless of dose were
recorded. In the case of referred patients, their referring physician
was reached to inquire about BP therapy. Post-operative follow
up was performed at 1, 2, 4, 8, and 10 weeks post-surgery, and
additional follow up visits were scheduled when necessary.
Phosphatemia (sPi) (mg/dL) was determined by colorimetry at
340nm using an autoanalyzer (Abbott Laboratories, Abbott Park,
IL, USA). Intra- and interassay coefficients of variation (CVs) were
0.5-5% and 0.3-0.6%, respectively. Bone alkaline phosphatase
(BAP) (IU/L) was determined by colorimetry at 520nm (optimized
Alkaline Phosphatase, Wiener SA) after precipitation of the bone
isoform with wheat germ lectin. Intra- and interassay CVs were
between 4-8% and 6-8%, respectively.
Levels of 25 hydroxyvitamin D (25OHD) (ng/mL) were
determined by competitive protein binding radioimmunoassay
(DiaSorin, Stillwater, MN, USA). Intra- and interassay CVs were
10% and 15%, respectively. C-terminal telopeptide of Collagen type
I (CTX) (ng/mL) was determined by enzyme immunoassay using
monoclonal antibodies (Osteometer BioTech, Herlev, Denmark).
Intra-assay CV was 6%.
Statistical analysis
Normality of the studied variables was tested using Shapiro- Wilk test. Homogeneity of variances was determined using Levene’s test. Student’s t test was used to compare the differences between the studied groups. All statistical analyses were performed using SPSS 19.0 for Windows (SPSS, Inc. Chicago, IL). Statistical significance was set at a value of p below 0.05 (p<0.05).
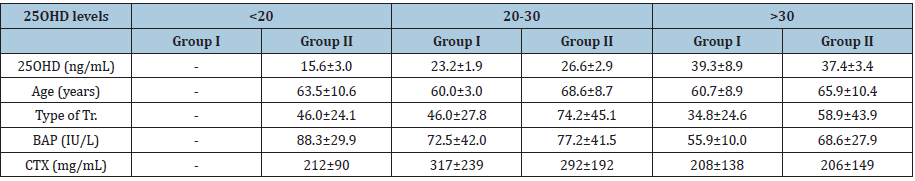
Result
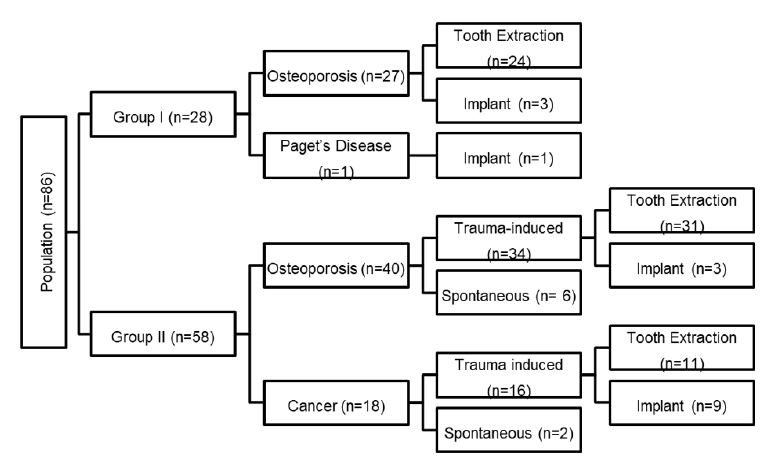
Data corresponding to the 86 women included in the study are shown in Figure 1. Twenty-seven of the 28 women in Group I were prescribed BPs for osteoporosis and one for Paget’s disease, whereas 40 of the 58 women in Group II were prescribed BPs for osteopenia/osteoporosis and 18 for cancer treatment. Twenty-four women in Group I had undergone tooth extraction and one had had dental implant surgery. Eighty-six percent of patients (n=50) in Group II, 34 of whom had osteoporosis and 16 had a neoplasm, developed BRONJ after tooth extraction or dental implant therapy; the remaining 8 patients in this group, six of whom had osteoporosis and 2 had a neoplasm, developed ONJ spontaneously.
Figure 1:Distribution of women in Groups I and II according to underlying disease, type of oral treatment, and occurrence of bisphosphonate related osteonecrosis of the jaw.
Comparison between women with and without BRONJ
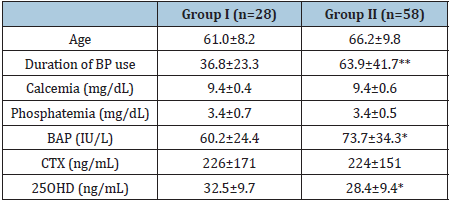
Comparison between groups showed women with BRONJ were older and duration of BP use was significantly longer (p=0.00001) in the group of women with BRONJ. No significant differences in serum calcium, phosphate, or CTX levels were observed between groups, whereas BAP levels were significantly higher and 25OHD levels were significantly lower in the group of women who developed BRONJ (p=0.040 and p=0.035, respectively) Table 1. In view of the highly significant differences in duration of use between groups I and II, all the above parameters were analyzed in a subset of patients with BRONJ whose duration of BP use was similar to that of Group I, i.e., women who did not develop BRONJ. As observed when comparing the entire Group I with Group II, mean age of the Group II subset was higher than mean age of Group I, but no significant differences in serum calcium, phosphate, or CTX levels were observed between the Group II subset and Group I (data not shown).
Table 1:Data of the women included in the study.
*P <0.05; **p<0.00001 : Group I vs. Group II
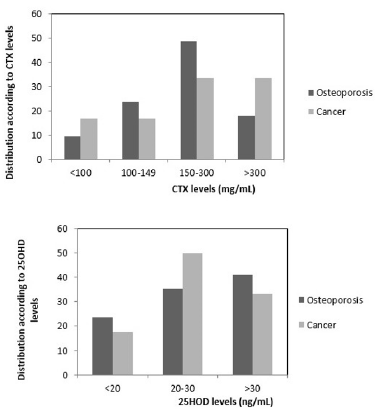
Figure 2:Percentage of women in Groups I and II according to CTX (A) and according to 25OHD levels (B).
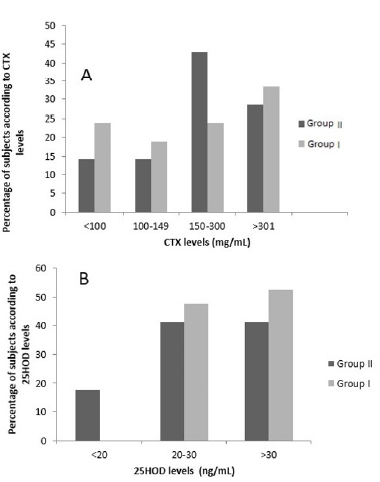
Distribution of patients in Groups I and II according to serum
CTX levels stratified as high, low, or minimal risk for BRONJ (8) is shown in Figure 2. No statistically significant differences in the
percentage of women with CTX levels between 100 and 149mg/
mL (14.3% and 19.0%) or between women with CTX >301 mg/
mL (28.5% vs. 33.4%) were observed between groups. However,
50% of women in Group II had CTX levels <100mg/mL (14.3%
vs. 23.8%), and the number of women with CTX levels in the 150-
300mg/mL range (42.9% vs. 23.8%) was twofold higher in Group II
as compared to Group I.
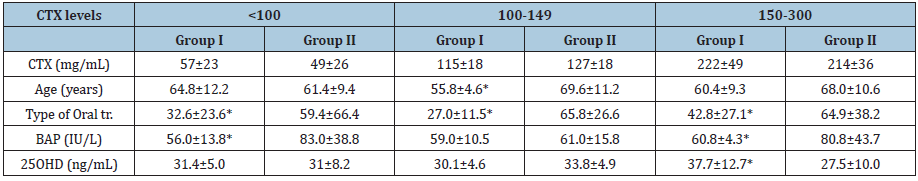
Comparison of duration of use and 25OHD levels between
groups according to range of CTX showed significantly lower
duration of use (p<0.0001) in each of the CTX ranges, and
significantly higher 25OHD levels (p<0.05) in the <100 and 150-
300 mg/mL CTX ranges in Group I Table 2.
Table 2:Data of women with and without BRONJ according to CTX levels.
Vitamin D status (sufficiency, insufficiency, deficiency) in each
group is shown in Figure 1. Whereas none of the subjects in Group I
had deficient VD levels, 18% of those in group II had 25OHD levels
below 20 ng/ml. The percentage of women with VD sufficiency/
insufficiency did not differ significantly between groups, and
accounted for 50% of women in Group I and 40% of those in Group
II.
Comparison of duration of use in patients with VD sufficiency
and insufficiency between groups, showed that duration of use was
significantly lower (p<0.001) in patients with VD sufficiency and
insufficiency in Group I. It was not possible to compare duration of
use in patients with Vitamin D deficiency since there were no cases
of Vitamin D deficiency in Group I Table 3.
Table 3:Data of women with and without BRONJ according to 25OHD levels.
Comparison of parameters according to onset of BRONJ showed
that CTX levels were significantly higher (264±169 vs. 189±87mg/
mL; p<0.05) in women who developed BRONJ after invasive
dental treatment as compared to those who developed BRONJ
spontaneously, and though the difference did not reach statistical
significance, duration of use (71.8±19.7 vs. 62.2±45.0 months) was
also longer (71.8±19.7 vs. 62.2±45.0 months) in the former sub-set
of women with BRONJ. No differences in age or in the remaining
biochemical parameters were observed between these two subsets.
As regards underlying disease prompting BP therapy, no
significant differences in serum calcium (9.3±0.4 vs. 9.7±0.8mg/
dL), phosphate (3.4±0.6 vs. 3.4±0.9mg/dL), BAP (73.1±31.2
vs. 79.3±38.5), or 25HOD (29.5±8.5 vs. 28.4±9.3mg/dL) levels
were observed between women with BRONJ receiving BPs for
osteoporosis and those receiving BPs for cancer treatment. Mean
CTX levels were higher in women with BRONJ receiving BPs for
cancer treatment than in those receiving BPs for osteoporosis
(225±121 vs. 291±222mg/mL, respectively), though the
difference did not reach statistical significance. Duration of use
was significantly higher in BRONJ patients with osteoporosis
than in those with a neoplasm (74.4±42.6 vs. 44.6±31.9 months,
respectively: p<0.01).
The percentage of patients with BRONJ receiving BPs for
osteoporosis and for cancer treatment according to serum CTX
and 25OHD values is shown in Figure 3. The percentage of
osteoporosis and cancer patients with CTX levels indicating high or
moderate risk for BRONJ was similar (33.3% vs. 33.4%), whereas
the percentage of cancer patients with CTX levels below 100mg/
mL was higher than that of women with osteoporosis (16.7% vs.
9.6%). The percentage of subjects with deficient/inadequate VD
levels was higher in the group of cancer patients (58.8% vs. 67.7%).
Comparison of parameters according to mode of delivery (oral
vs. intravenous administration) showed that serum Ca, BAP, CTX, and 25OHD levels were higher and serum phosphate levels were
lower in women receiving intravenous BPs, though only BAP levels
differed significantly (70±5 vs. 97±10 IU/L; p<0.05).
Figure 3:Percentage distribution of women with osteoporosis and cancer according to CTX and 25OHD levels (A and B, respectively).
Discussion
Serum CTX levels were not found to predict risk for ONJ. Of
note, a high percentage of patients on long-term BP treatment,
including those who developed BRONJ and those who did not, had
inadequate VD status.
The degree of bone turnover can be assessed using biochemical
markers of bone remodeling, which include osteoblastic bone
formation and osteoclastic bone resorption markers. When bone
formation and resorption are coupled and coordinated, it is sufficient
to determine one in order to assess total bone remodeling. CTX is
currently considered one of the most specific and sensitive markers
of bone resorption [16,17]. CTX levels decrease dramatically one
or two months after initiation of oral or intravenous antiresorptive
BP treatment. This decrease prevents bone mass loss, and the risk
of fracture therefore decreases. However, suppression of bone
remodeling for a prolonged period of time can cause adverse
secondary effects such as atypical fractures and BRONJ [3].
Bisphosphonate-related osteonecrosis of the jaw is a
complex condition of multifactorial origin, involving a number of
mediators. The marked decrease in bone remodeling resulting
from BP treatment is considered one of the key risk factors for the
development of this disease [18]. However, there is a wide range of
individual variability among patients receiving the same dose and
even the same BP [14], likely due to genetic susceptibilities [19].
Biochemical determination of CTX levels reflects total bone
resorption activity, rather than resorption at a specific skeletal site.
In this regard, it must be kept in mind that the bone remodeling
process in the jaws differs from that of the axial skeleton, and the
jaws also differ in their response to aminobisphosphonate therapy.
It has therefore been posited that BRONJ cannot develop without
significant suppression of bone remodeling. Should the latter occur,
minor trauma as is tooth extraction could trigger the disease. In this
regard, Mark RE et al. concluded that the risk of BRONJ in patients
on BP therapy for more than three years was high when their CTX
levels were <100pg/mL, moderate with CTX levels 100 to 150pg/ mL, and minimal with CTX values 150 a 299pg/mL. Based on the
above, the 2009 update of the AAOMS guidelines recommended
discontinuing BP therapy for three months prior to dental surgery
(bisphosphonate holiday) in order to minimize risk of developing
BRONJ [20]. However, due to their high affinity for hydroxyapatite,
aminobisphosphonates remain in the bone microenvironment
over long periods of time, with the more potent BPs remaining
in the body up to10 years. It would therefore be unlikely for the
concentration of BP in the bone to change at such short times. A
number of researchers [21-23] have also lent support to the utility
of CTX levels as a potential risk predictor of BRONJ, though more
recent studies seem to challenge this conclusion [22,24]. In their
2011 report, the American Dental Association Council on Scientific
Affairs (ADAC) concluded that there was not sufficient evidence
to consider the different levels of biochemical markers of bone
remodeling as risk predictors for developing BRONJ [25]. The 2014
update of the AAOMS further supported the lack of validation of a
systemic marker of bone remodeling to assess the risk for BRONJ
[3].
In agreement with the above reports, we found no difference
in average CTX levels or in the percentage of women with CTX
levels in the low, moderate, and high risk ranges between patients
with and without BRONJ. CTX levels were only found to differ
within the group of BRONJ patients, and were lower in those who
developed BRONJ spontaneously than in those who developed
BRONJ after invasive dental treatment. Fifty percent of women with
spontaneous BRONJ but only 25% of those with dental treatmentinduced
BRONJ had CTX levels below 150mg/mL. According to
the classification proposed by Mark RE et al., therefore, 50% and
75% of patients with spontaneous and trauma-induced BRONJ
respectively were at minimal risk of developing the disease, though
100% of these patients in fact developed BRONJ [8].
Other additional risk factors for BRONJ that would affect
CTX levels include age, invasive oral treatment, type of BP, dose,
and duration of use [26]. Both groups of women treated with
BPs were similar in age, and all underwent oral treatment (tooth
extraction or implant surgery), but differed in duration of use and
type of BP. Although there were no available data on the dose, it is
well documented that the dose used to treat neoplasms or active
Paget’s disease is markedly higher than the dose used to treat
osteopenia/osteoporosis. The mode of delivery also differs with
regard to the aforementioned conditions: neoplasms and Paget’s
disease are usually treated with intravenously administered
BPs, whereas osteoporosis/osteopenia are treated with oral BPs.
Intravenous administration of BPs has been associated with high
risk of developing BRONJ [27]. Nevertheless, our results showed
no difference in mean CTX levels or in the percentage of women
with CTX levels <150mg/mL when comparing women receiving
intravenous and oral BPs (data not shown).
Because BPs deposit in the bone, the quantity of the drug
that remains in the bone microenvironment will depend on
duration of treatment. In the present study, duration of treatment with aminoBPs was two-fold higher in the group of women who
developed BRONJ than in the group who did not. In addition, our
results showed that duration of use was shorter among the cancer
patients than the osteoporosis patients who developed BRONJ.
Despite these differences, according to reports in the literature, CTX
levels stabilize after six weeks of treatment and are independent
of treatment duration [23]. In line with these findings, our results
showed that duration of treatment did not affect mean CTX levels
or the percentage of women with CTX levels below 150mg/mL.
The type of BP also differed. Intravenous zoledronate was the
treatment of choice for neoplasms but zoledronate was not used
for osteoporosis treatment (50% vs. 0%, respectively). Conversely,
oral alendronate was not used to treat neoplasms but was the
choice treatment for osteoporosis (0% vs. 38%, respectively). The
observed difference in the type of aminoBP and mode of delivery
did not affect mean CTX levels or the percentage of women with
CTX levels below 150mg/mL (data not shown).
It must be pointed out that although the present results do not
lend support to the utility of serum CTX levels as predictor of risk
for developing BRONJ, it is important to maintain the levels of this
bone marker within reference range in order to prevent potential
adverse effects of BP therapy.
Treatment with BPs can induce hypocalcemia and subsequently
hyperparathyroidism [28]. According to reports in the literature,
hyperparathyroidism may be involved in the complex etiology
of BRONJ, since patients who develop BRONJ have persistent
hypocalcemia and secondary hyperparathyroidism during the
period before the onset of BRONJ [29]. Hellstein JW et al. [25] found
that patients with higher immunoreactive PTH levels prior to or
during BP treatment were more likely to develop BRONJ than those
showing normal iPTH before PB treatment. Because PTH levels
were not determined in all the women included in the present study,
we were not able to evaluate the relation between PTH levels prior
to BP treatment and development of BRONJ. Nevertheless, none of
the women studied here had hypocalcemia, a specific marker of an
increase in the parathyroid secretion.
Vitamin D deficiency has been considered a possible risk factor
for BRONJ. In this regard, Hokugo A et al. [27] found VD deficientrats
treated with zoledronate to be at higher risk for BRONJ [27].
Clinical studies conducted by Beddoni A et al. showed a strong
association between VD deficiency and risk for developing BRONJ
[15]. Vitamin D deficiency and hyperparathyroidism are associated
since insufficient 25OHD levels increase PTH levels through loss
of negative feedback of VD on parathyroid hormone synthesis
and secretion [27,30]. It is therefore relevant to consider whether
increased iPTH levels, VD insufficiency, or both combined may
play a role in the onset of BRONJ. As mentioned above, iPTH levels
were not assessed in the present study. Nevertheless, given that the
mean 25OHD levels of women with BRONJ were lower than those
of women without BRONJ, it could be hypothesized that PTH levels
might have been higher, though still within the reference range, at
least in women with 25OHD levels in the insufficiency range. The lower mean 25OHD levels observed in the group of women with
BRONJ are accounted for not only by the higher percentage of
women with inadequate VD status in this group but also by the fact
that approximately 20% of women in this group had VD deficiency.
Badros A et al. [31] found that 40% of patients with multiple
myeloma had VD deficiency (<14.4ng/mL) and 35% had VD
insufficiency (14.4 a 30ng/mL) [31]. Lowe LC et al. [32] reported
that 30.2% patients with breast cancer had VD insufficiency [32].
Multiple myeloma and cancer patients are usually treated with high
doses of BPs, so that these patients on long term treatment with
BPs could develop BRONJ. Although each of the aforementioned
authors reported the percentage of multiple myeloma and cancer
patients with vitamin D insufficiency/deficiency, respectively,
they did not provide information regarding the presence of BRONJ
among the patients they included in their study. It could be thought
that their study population may have included subjects receiving
high doses of BPs and who also had VD insufficiency/deficiency,
and were therefore at risk for developing BRONJ. We found 45% of
cancer patients who developed BRONJ in the insufficiency range.
However, because 100% percent of the BP-treated cancer patients
in our study developed BRONJ, we could not establish comparisons
with BP-treated patients without BRONJ [33].
It is well documented that bone metabolism is directly affected
by BP therapy, though in a complex manner on account of the
number of mediators involved. It is therefore important to maintain
the different mediators within reference ranges for the proper
pharmacological action of BPs. In this regard, there is consensus
that the therapeutic efficacy of BPs in decreasing fracture risk is
suboptimal under conditions of VD deficiency [2]. In the present
study, however, 60% of BP-treated women who developed BRONJ
and 50% of those who did not were in the VD insufficiency range.
To conclude, the present results do not allow confirming the
utility of serum CTX levels as predictor of risk for developing BRONJ.
In addition, the high percentage of women with VD deficiency who
developed BRONJ suggests the importance of determining the
physiological status of bone metabolism in order to adjust calcium,
VD, and PTH levels on account of the potential relation between
these parameters and the development of BRONJ
Acknowledgement
The present study was part of the Doctoral Thesis Research work of Silvana Noemi Picardo, PhD, DDS, entitled “Osteonecrosis of the Jaw in Patients undergoing Long-Term Treatment with Bisphosphonates: Incidence and Associated Characteristics” (“Osteonecrosis Maxilar en Pacientes Tratados en Forma Crónica con Bifosfonatos: Incidencia y Características Asociadas”) and was partly funded by the UBA and CONICET.
References
- Compston JE (1994) The therapeutic use of bisphosphonates. BMJ 309(6956): 711-715.
- Mastaglia SR, Pellegrini GG, Mandalunis PM, Chaves MMG, Friedman SM, et al. (2006) Vitamin D insufficiency reduces the protective effect of bisphosphonate on ovariectomy-induced bone loss in rats. Bone 39(4): 837-844.
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, et al. (2014) American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg 72(10): 1938-1956.
- Advisory task force on bisphosphonate-related ostenonecrosis of the jaws (2007) American association of oral and maxillofacial surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 65(3): 369-76.
- Aghaloo T, Hazboun R, Tetradis S (2015) Pathophysiology of osteonecrosis of the jaws. Oral Maxillofac Surg Clin North Am 27(4): 489-496.
- Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19(6): 733-759.
- Sebba AI, Broy S, Kohles JD, Weissman P (2008) Rapid suppression of bone resorption marker levels with ibandronate therapy in a bisphosphonate-naïve population. J Clin Densitom 11(3): 417-423.
- Marx RE, Cillo JE, Ulloa JJ (2007) Oral bisphosphonate-induced osteonecrosis: Risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg 65(12): 2397-2410.
- Bagan JV, Jimenez Y, Gomez D, Sirera R, Poveda R, et al. (2008) Collagen telopeptide (serum CTX) and its relationship with the size and number of lesions in osteonecrosis of the jaws in cancer patients on intravenous bisphosphonates. Oral Oncol 44(11): 1088-1089.
- Lee CY, Suzuki JB (2010) CTX biochemical marker of bone metabolism. Is it a reliable predictor of bisphosphonate-associated osteonecrosis of the jaws after surgery? Part II: A prospective clinical study. Implant Dent 19(1): 29-38.
- Baim S, Miller PD (2009) Assessing the clinical utility of serum CTX in postmenopausal osteoporosis and its use in predicting risk of osteonecrosis of the jaw. J Bone Miner Res 24(4): 561-574.
- Sawatari Y, Marx RE (2007) Bisphosphonates and bisphosphonate induced osteonecrosis. Oral Maxillofac Surg Clin North Am 19(4): 487-498.
- Zak M, Spina AM, Spinazze RP, Perkinson WL, Spinazze DJ (2007) Bisphosphonates and the dental patient: Part 2. Compend Contin Educ Dent 28(9): 510-515.
- Khosla S (2008) Oral bisphosphonate-induced osteonecrosis: Risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg 66(6): 1320-1321.
- Bedogni A, Saia G, Bettini G, Tronchet A, Totola A, et al. (2012) Osteomalacia: The missing link in the pathogenesis of bisphosphonate-related osteonecrosis of the jaws? Ooncologist 17(8): 1114-1119.
- Kwon YD, Lee CY, Hong SO, Lee YA, Ohe JY, et al. (2016) Bisphosphonate related osteonecrosis of the jaws (BRONJ) in osteoporotic males. Springerplus 5(1): 1468.
- Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS et al. (2000) Serum CTX: A new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int 66(2): 100-103.
- Cheng A, Mavrokokki A, Carter G, Stein B, Fazzalari NL, et al. (2005) The dental implications of bisphosphonates and bone disease. Aust Dent J 50(4 Suppl 2): S4-S13.
- Nicoletti P, Cartsos VM, Palaska PK, Shen Y, Floratos A, et al. (2012) Genomewide pharmacogenetics of bisphosphonate-induced osteonecrosis of the jaw: The role of RBMS3. Oncologist 17(2): 279-287.
- Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, et al. (2009). American association of oral and maxillofacial surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg 67(5 Suppl): 2-12.
- Ardine M, Generali D, Donadio M, Bonardi S, Scoletta M, et al. (2006) Could the long-term persistence of low serum calcium levels and high serum parathyroid hormone levels during bisphosphonate treatment predispose metastatic breast cancer patients to undergo osteonecrosis of the jaw? Ann Oncol 17(8): 1336-1337.
- Kim JW, Kong KA, Kim SJ, Choi SK, Cha IH, et al. (2013) Prospective biomarker evaluation in patients with osteonecrosis of the jaw who received bisphosphonates. Bone 57(1): 201-215.
- Pasoff M (2013) C-terminal cross-linking telopeptide as a serologic marker for bisphosphonate-related osteonecrosis of the jaw: Review of 2 cases. J Can Dent Assoc 79: d51.
- Pasoff M (2013) C-terminal cross-linking telopeptide as a serologic marker for bisphosphonate-related osteonecrosis of the jaw: Review of 2 cases. Journal Canadian Dental Association 79: d51.
- Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, et al. (2011) Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: Executive summary of recommendations from the American dental association council on scientific affairs. J Am Dent Assoc 142(11): 1243-1251.
- Hutcheson A, Cheng A, Kunchar R, Stein B, Sambrook P, et al. (2014) A C-terminal crosslinking telopeptide test-based protocol for patients on oral bisphosphonates requiring extraction: A prospective single-center controlled study. J Oral Maxillofac Surg 72(8): 1456-1462.
- Hokugo A, Christensen R, Chung EM, Sung EC, Felsenfeld AL, et al. (2010) Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res 25(6): 1337-1349.
- Berruti A, Dogliotti L, Tucci M, Tarabuzzi R, Fontana D, et al. (2001) Metabolic bone disease induced by prostate cancer: Rationale for the use of bisphosphonates. J Urol 166(6): 2023-2031.
- Ardine M, Generali D, Donadio M, Bonardi S, Scoletta M, et al. (2006) Could the long-term persistence of low serum calcium levels and high serum parathyroid hormone levels during bisphosphonate treatment predispose metastatic breast cancer patients to undergo osteonecrosis of the jaw? Ann Oncol 17(8): 1336-1337.
- Baldock PA, Thomas GP, Hodge JM, Baker SU, Dressel U, et al. (2006) Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res 21(10): 1618-1626.
- Badros A, Goloubeva O, Terpos E, Milliron T, Baer MR, et al. (2008) Prevalence and significance of vitamin D deficiency in multiple myeloma patients. Br J Haematol 142(3): 492-494.
- Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, et al. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer 41(8): 1164-1169.
- Lehrer S, Montazem A, Ramanathan L, Minsley MP, Pfail J, et al. (2008) Normal serum bone markers in bisphosphonate-induced osteonecrosis of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106(3): 389-391.
© 2021 Rey EA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






