- Submissions

Full Text
Global Journal of Endocrinological Metabolism
Molecular Pathways of, and the Future Therapeutic Implications for Atopic Dermatitis: A Review
ASM Giasuddin1*, Shafiqul Islam2, Khadija Akther Jhuma3 and AM Mujibul Haq4
1 Department of Biochemistry & Immunology, Medical Research Unit (MRU), Bangladesh
2 Department of Dermatology, Medical College for Women & Hospital Member Secretary, Medical Research (MRU), Bangladesh
3 Department of Biochemistry, Medical College for Women & Hospital Member Secretary, Medical Research Unit (MRU), Bangladesh
4 Department of Medicine, Medical College for Women & Hospital Founder Principal & Chairman Projects, MHWT Chairman, Medical Research Unit (MRU), Bangladesh
*Corresponding author: ASM Giasuddin, Department of Biochemistry & Immunology, Medical Research Unit (MRU), Bangladesh
Submission: Arpril 02, 2019; Published: April 09, 2019

ISSN 2637-8019Volume3 Issue1
Abstract
No available treatments can provide long-term remission for patients with moderate to severe Atopic Dermatitis (AD) creating a large unmet need for effective systemic treatment. The important factors and related mechanisms for AD are the following:
a) Genetic
b) Neurohumoral
c) Skin barrier dysfunction and
d) Immunological mechanisms
The cellular interactions, molecular events and pathways for the pathogenesis of AD was integrated into a hypothesis and reported recently. As the new insights into the immune and molecular pathways of AD increase, a variety of experimental agents, particularly biological agents that target pathogenic molecules bring promise of safe and effective therapeutics. Some of the most promising biological therapies that are in development or in clinical trials are based on principles as the following: Barrier repair, Allergen specific immunotherapy, Targeted Immunomodulating Therapies (TIT) (Anti-IgE therapy, Anti-CD20, Inhibition of T-cell responses, Th2-cell inhibition strategies/ Anti IL-4 therapies, Anti IL-5 strategies, Anti IL-31), Targeting Th22, Targeting Th17/IL-12/IL-23 pathway, Recombinant IFN-y, Anti IL-6R, Anti TNF agents, Phosphodiesterase inhibitors, PPAR-gamma agonists.
All these biological therapies are at different phases of clinical trials. These biological agents would potentially hold great promise for the treatment of AD if they can offer advantages, i.e. low toxicity, good efficacy, improved patient compliance via given weekly/biweekly/and even monthly administration, reduction of disease activity, relapse prevention, etc. In Summary, the recent advances in understanding of the immunopathogenic mechanisms provide an opportunity for development of biological therapies directed at pathways driving AD. This is possibly the beginning of an exciting era in AD therapeutics with impending availability of drugs having low toxicity and increased patients’ compliance. These expected drugs will not only treat this disease, but also prevent the development and relapse of new skin lesions. In this article, attempts have been made to provide an updated account of these new possibilities.
Keywords: Atopic dermatitis; Eczema; Biologics; Therapeutics; Cytokines; Anti-cytokines
Introduction
Atopic Dermatitis (AD) is a common, chronic, relapsing inflammatory skin disease affecting increasing number of people up to 25% of children and up to 3% of the adult population [1-3]. AD, also named eczema in some countries, is considered as the most common, itchy and relapsing inflammatory skin condition. Together with asthma and allergic rhinitis, it constitutes the “Atopic Triad”. Approximately 80% of patients have a personal or family history of atopy, associated with high serum Immunoglobulin E (IgE) level and/or elevated eosinophil count referred to as extrinsic AD in contrast to intrinsic AD that lacks these characteristics [4,5]. Despite its increasing prevalence worldwide, no available treatments can provide long-term remission for patients with moderate to severe AD, creating a large unmet need for effective systemic treatment [6- 8]. Much progress has been made in the understanding of its genetic background and pathophysiology through studies in genetics, epidemiology and immunology. These studies have provided new important insight of the complex puzzle and dramatically changed our view on the pathogenesis, its natural history and the future ways to control the disease AD in the context of the so-called atopic march [2,9-12].
Pathogenetic Mechanism
Among the various factors involved in the pathogenesis of AD, the important ones are the following:
a) Genetic factors
b) Neurohumoral factors
c) Skin barrier dysfunction and
d) Immunological mechanisms [2]
Genetic factors
The importance of genetic factors in AD is underlined by the finding that a positive parental history is the strongest risk factor for AD; the incidence rate is double if AD is present in one parent and tripled if both parents are affected. In the modern era of genomics, linkage analysis and association studies have greatly contributed to our understanding on the genetic background of AD [8,9]. The important chromosomal region harboring possibly several important genes is 1q21.
This area includes most of the genes regulating the epidermal homeostasis, the epidermal differentiation complex (EDC). The recent demonstration of loss-of-function mutations of the profilaggrin/filaggrin gene, a key protein in terminal differentiation of the epidermis, can be considered as a breakthrough [2,10,11]. It is expected that other yet-to-be-defined genetic variants from epidermal structures such as those localization in the EDC on chr. 1q21 may also play a role in these phenomena [13,14]. The other set of candidate genes includes the numerous structures related to immunological mechanisms operative in AD. For example, on chromosome 5q31-33, the locus containing genes for the TH2 cytokines interleukin (IL)-3, IL-4, IL-5, IL 13, and granulocyte macrophage colony stimulation factor (GM-CSF) has been suggested. Further studies identified variants of the IL-13 encoding region, functional mutations of the promoter region of the chemokine RANTES (Regulated on Activation, Normal T cell Expressed and Secreted) (17q11) and gain-of function polymorphisms in the alpha subunit of the IL-4 receptor (16q12) [14]. The disbalance between TH1 und TH2 –immune responses in AD may be elucidated by the detection of polymorphisms of the IL-18-gene, resulting in TH2 predominance [15].
Neurohumoral factors
Neuropeptides and neurotropins mediate different actions such as vasodilatation, oedema, itch and pain or sweat gland secretion and have a minor ability to regulate T-cell activation [16,17]. They can be detected in blood and within the epidermal nerve fibres in close association with mast cells or epidermal Langerhans cells, suggesting a tight link between the immune system and the nervous system. Recent studies have documented increased levels of nerve growth factor and substance P in plasma of AD patients while growth factor, detected recently in sera and plasma of patients with AD, enhances the survival of eosinophils, increasing their chemotactic response in vitro [16].
Skin barrier dysfunction
One of the major hallmarks of AD is xerosis which affects lesional and non-lesional skin areas as due to increased trans epidermal water loss. It may favour the penetration of high-molecular weight structures such as allergens, bacteria, and viruses [17]. Several mechanisms have been postulated:
a) A decrease in skin ceramides, serving as the major waterretaining molecules in the extracellular space,
b) Alterations of the stratum corneum pH [18]
c) Over expression of the chymotryptic enzyme (chymase),
d) Defect in Filaggrin as well as molecules of the EDC the protein family (see genetic factor)
Immunological mechanism: Both the components of the immune system i.e.
a) innate immunity and
b) b) adaptive/acquired immunity, are important in the pathogenesis of AD.
Innate immunity: The innate immunity system of the epidermis presents the first line defense against cutaneous infections. Once the epidermis is invaded by micro-organisms, anti-microbial peptides are activated and form part of the defense system [19]. AD skin is characterized by a significant decrease in expression of anti-microbial peptide, explaining the susceptibility of AD patients for bacterial infections [2,20]. The innate skin defence system of patients with AD may be further reduced by the deficiency of dermcidin-derived antimicrobial peptides in sweat, which correlates with infectious complications [21].
Acquired immunity: A predominant TH2 disbalance with increased IgE levels and eosinophilia is widely accepted in the pathogenesis of AD [22-24]. The production of TH2 mediated cytokines, notably IL-4, IL-5, and IL-13, can be detected in lesional and non-lesional skin during the acute phase of disease. IL-4 and IL- 13 are implicated in the initial phase of tissue inflammation and may mediate an isotype switching to IgE synthesis, and up regulation expression of adhesion molecules on endothelial cells. IL-5 increases the survival of eosinophils and a systemic eosinophilia with an increase of the eosinophil cationic protein (ECP) correlate to disease severity [25]. Although TH2 mediated cytokines seem to be predominant in the acute phase of AD they are less important during its chronic course. The maintenance of chronic AD involves further on the production of the TH1 like cytokines IL-12 and IL-18, as well as several remodeling-associated cytokines such as IL-11, IL-17 and TGF-β1 [26]. Role of different chemokines have gained interest in the pathogenesis of AD.
Many chemokines like TARC/CCL17, PARC/CCL18, MDC/ CCL22, and CCL1 seem instrumental in the development of acute and chronic lesions [2,22]. C-C chemokines (RANTES, exotaxin, etc) contribute to the infiltration of macrophages, eosinophils, and T-cells into acute and chronic AD skin lesions. Dendritic cells (DC) are highly specialized professional antigen presenting cells and are essential for the allergen uptake and its presentation to T-cells in the context of primary and secondary immune responses. The role DC in AD has been extensively discussed elsewhere [27,28]. Lesional and normal skin of patients with AD is highly colonized with toxins-production staphylococcus aureus (S. aureus) [29].
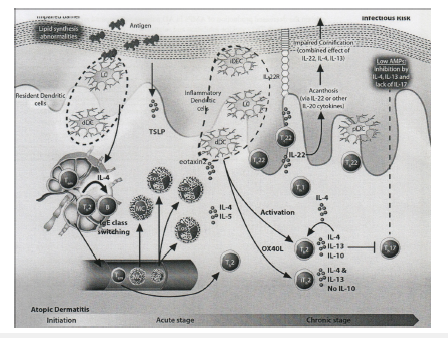
This colonization is due to the decreased production of antimicrobial peptides which are down-regulated by the particular inflammatory micro-milieu in AD.30 Interestingly, S. aureus enterotoxines A (SEA), B (SEB), C (SEC) and D (SED) gained increasing importance in the pathogenesis of AD since they exhibit a large spectrum of biological activities including the induction of a specific (IgE) sensitization, acting as super antigens and altering the function of Treg [2,6]. These cellular interactions, molecular events and pathways for the pathogenesis of AD has been integrated into a hypothesis and reported by Guttman-Yassky et al (Figure-1) [6,31].
Figure 1:The immunopathogenesis of Atopic Dermatitis (AD).
The disease has three main phases, i.e. initiation, acute and chronic stages. Defects in the epidermal barrier lead to the penetration of the skin by epicutaneous antigens, which in turn encounter Langerhans and dermal dendritic cells that activate Th2 cells and IL-4 and IL-13 production. These cytokines result in two major effects: IgE class switching and increased Th2 cell survival having several direct effects on the epidermis. These include inhibition of anti-microbial peptide (AMP) production, and impaired epidermal differentiation. In addition, the inflammatory mediators of Th2 T-cells and DCs induce peripheral eosinophils and mast cells. Also, of significance is an increase in Th22 cells in AD skin; this subset produces IL-22, which is most significantly increased in chronic AD skin. IL-22 inhibits terminal differentiation and induces epidermal hyperplasia, which is an important characteristic of chronic disease. Thus, the barrier defect in AD most likely results from a combined effect of Th2 and Th22 cytokines. Similarly, to T-cells, there is a progressive increase in dendritic cells and Langerhans cells from non-lesional through chronic AD Adapted from: Guttman-Yassky et al. [6,31].
Biological therapeutics in development
One might hypothesize that in AD epidermal reaction may be largely restored to normal with selective immune suppression. A recent study of AD patients that were treated with Narrow Band (NB) UVB therapy, showed that reversal of clinical disease activity was associated with reversal of the epidermal pathology including reduction in epidermal thickness and expression of proliferation markers [32]. As the new insights into the immune and molecular pathways of AD increase, a variety of experimental agents, particularly biological agents, that target pathogenic molecules bring promise of safe and effective therapeutics for long term use. Some of the emerging and most promising biological therapies that are in development or in clinical trials are based on principles as the following: Barrier repair, Allergen specific immunotherapy, Targeted Immunomodulating Therapies (TIT) (Anti-IgE therapy, Anti-CD20, Inhibition of T-cell responses, Th2-cell inhibition strategies/ Anti IL-4 therapies, Anti IL-5 strategies, Anti IL-31, Targeting TSLP), Targeting Th22, Targeting Th17/IL-12/IL-23 pathway, Recombinant IFN-y, Anti IL-6R, Anti TNF-α agents, Phosphodiesterase inhibitors, PPAR-gamma agonists. However, further developments in future targeting and inhibiting cytokines, chemokines and signal transduction are strong possibilities. [31,33-38]. All the above stated biological therapies are at different phases of clinical trials. However, trials showed effectiveness of these treatments differentially in only subset of patients.
Barrier repair: Skin barrier function is linked to the innate immune system. The therapeutic strategies which improve innate immunity might ultimately lead to repair of the epidermal barrier. A meta-analysis of trials of probiotics, however, did not show that they benefitted patients with AD. The findings that vitamin D3 supplements up regulated production of AMPs should be confirmed in larger population of patients with AD. Topical protease inhibitors were also being tested for treatment of AD, although there was no evidence for their benefit. Recently, another approach aims to restore and increase the expression of epidermal differentiation proteins, e.g. filaggrin and loricrin, in order to repair the barrier [31,39-44].
Allergen specific immunotherapy: Double-blinded, multicentre, randomized trials with house dust mites have been reported to significantly improve the eczema in patients with AD who are sensitized to house dust might allergen. Allergen specific immunotherapy represents the important causative therapeutic approach. With regard to AD only limited, and often contradictorily information is available. A study, re-examining the efficacy of a subcutaneous immunotherapy (SCIT) in atopic patients sensitized to house dust mites (HDM), demonstrated effectiveness improving eczema and allergic sensitization to HDM and reduction of topical corticosteroids required to treat eczema. Oral immunotherapy (OIT) protocols are also being optimized for patients with a history of AD and food allergy [31,45-47]. Further studies are needed to evaluate the comparative benefits of SCIT and OIT in AD patients in the near future.
Targeted Immunomodulating Therapy (TIT)
TIT (Anti-IgE therapy): Although the role of IgE in the pathogenesis of AD is not clear, omalizumab, a humanized IgG1 monoclonal antibody against IgE, has been tested in AD mainly in patient’s refractory to conventional therapy. Omalizumab has not been observed to have consistent significant clinical effects in most patients with AD, despite its ability to down regulate FceRI on DCs. This may be in part due to the very high serum IgE levels which may be difficult to neutralize in AD as compared to asthmatics who have lower serum IgE. Alternatively, IgE might only play a secondary role to the primary cellular mechanisms in atopic dermatitis, in which case the therapeutic efficacy of omalizumab would be limited. Overall, this highlights the heterogeneity and complexity of allergic diseases suggesting that more work is needed to characterize AD patients into defined immunologic profiles and phenotypes which may benefit from specific biological therapies [7,31,47].
TIT (Anti-CD20 therapy): Small studies of rituximab, an antibody against CD20 that depletes B cells which was developed for hematologic disorders, have had contradictory results in patients with AD. One study showed that treatment with rituximab resulted in a rapid and sustained decrease of skin inflammation in patients with AD, suggesting a possible role for B cells in its pathogenesis. However, IgE levels remained unchanged during treatment. Further studies are required to determine whether reagents that inhibit or deplete B cells might be used to treat AD.31,48,49
TIT (Anti IL-4 therapy): Few inhibitors of the IL-4R are currently available. An inhibitor of IL-4R signaling (pitrakinra /pascolizumab) that competitively binds to IL-4RA to inhibit binding of IL-4 and IL-13 has shown efficacy in trials of patients with asthma but has not been tested for patients with AD. Of great interest is REGN668, an anti-IL-4R antibody currently evaluated in clinical trials for AD and eosinophilic asthma (phase 2 in eosinophilic asthma and completing phase 1 in AD). The clinical impact of the Th2 cytokines IL-4 and IL-13 on atopic dermatitis has recently been documented in clinical studies with dupilumab, a monoclonal antibody, which blocks the IL-4/IL-13 receptor and in fact, dupilumab has been already approved in 2017 for use in AD [31,50-52].
TIT (Anti IL-5 strategy): Eosinpphils are important mediators of the inflammatory process in AD, so agents that block IL-5 might be developed as therapeutics. However, mepolizumab, a fully humanized monoclonal antibody against IL-5, reduced blood and tissue eosinophilia but did not show clinical efficacy in patients with moderate to severe AD. Mepolizumab reduced the numbers of eosinophils in patients with asthma, but had no effects on T-cell responses, arguing against its role in treatment of AD [31,53,54].
TIT (Anti IL-31 therapy): IL-31 is a cytokine produced by Th2 cells that is believed to promote itching in AD. Recently it has been shown that IL-31 is one of the markers that are most significantly increased, possibly correlating with the increased pruritis in acute AD. Treatment with anti-IL-31 might hold promise for eczema as well as other itch-related dermatoses. Therapeutics that target IL- 31 are under development and in phase I clinical trials [31,55,56].
TIT (Anti Th22/Anti IL-22 therapy): The recently described Th22 T-cell subset was shown to have correlation with disease activity in AD. Th22 cells and IL-22 are increased in patients with acute as well as chronic AD. These were taken as high indication that IL-22 might be a therapeutic target for patients with AD [31,57,58].
TIT (Th17/IL-12/IL-23 pathway): As the levels of IL-17 and its related factors are increased in patients with AD, agents directed against IL-17 cytokine or its receptor (such as Ixekinumab, Brodalumab, AIN457) and/or IL-23 (such as MK-3222) might be effective for patients with acute exacerbations of AD. Anti-p40 (such as Ustekinumab/Stelara) might also be effective by targeting multiple pathways involved in AD, including Th17 and Th22 pathways [31,57,59,60].
TIT (Recombinant interferon gamma): Th1 subset of T cells from AD patients has been shown to produce lower levels of IFN-G. Administration of recombinant IFN-G to patients with AD might restore the balance of Th1 and Th2 cell responses, and lower IgE production. However, trials showed effectiveness of this treatment in only a subset of patients and did not reduce levels of IgE. This treatment might still have a potential role in patients with concomitant skin infections such as herpes simplex, and molluscum contagiosum [31,61].
Anti-IL-6R therapy: Anti IL-6R treatment with Tocilizumab seemed to be promising. Tocilizumab is an IL-6 receptor (IL-6R) antagonist approved by FDA, USA in 2008. Recently, this antagonist was used to treat 3 AD patients, refractory to other treatments, including cyclosporine A. All patients showed significant clinical improvement, with more than 50 % reduction in the Eczema Area and Severity Index (EASI) score. However, bacterial infections were observed in two of the three patients. Further studies are needed to evaluate the potential efficacy and the safety of Tocilizumab in patients with severe AD [31,37].
Anti TNF-α therapy: Anti TNF-α agents have been successful in treating psoriasis probably because TNF and its synergistic interaction with IL-17 mediate the pathogenesis of psoriasis. Furthermore, TNF-α antagonists inhibit the pathogenic Th1- and Thl7-cell responses that contribute to psoriasis. However, a pilot study of the effects of the TNF-α antagonist, infliximab, in patients with moderate-to-severe AD, and another study of etanercept in two children, demonstrated disappointing results, possibly because TNF-induced inflammatory responses have only a minor role in AD. Although clinical improvement was obtained, the patients did not show sustained responses [31,38,62].
PPAR-gamma agonists (Thiazolidinediones): Thiazolidinediones activate the nuclear receptor, PPAR-y, which is expressed on adipocytes and immune cells. Activation of PPAR-Y decreases production of pro-inflammatory cytokines (i.e. TNF-α and IL-6) and also increases responses to insulin. These agents were first approved for treatment of diabetes mellitus and recently also explored for inflammatory skin diseases, such as psoriasis and AD. Few clinical studies with systemic PPAR-y agonist, Rosiglitazone, led to clinical improvement and reduced number of flares in few patients with recalcitrant AD, and topical PPAR-α agonist showed beneficial clinical effects in pediatric AD. Both PPAR-y and PPAR-α are thought to have anti- inflammatory and barrier-normalizing properties and might be useful for treatment of established AD skin lesions and also possibly for prevention of new lesions [31,63].
Chymase inhibitor therapy: Chymase is an inflammatory molecule produced by mast cells which might be associated with AD. After being successful in mice, clinical trials were initiated in humans with chymase inhibitor, SUN-C8257, and currently, an oral chymase inhibitor SUN 13834 is in Phase II clinical trial in AD [31,64].
Conclusion
There has been a growing trend forwards the use of targeted therapies in treating AD in recent years. The systemic biological agents are potentially great promise for the treatment of AD if they can offer the following advantages: low toxicity, good efficacy, improved patient compliance via given weekly/biweekly/and even monthly administration, reduction of disease activity, relapse prevention, etc. Since there is a very close relationship between elucidation of molecular disease pathways and development of targeted systemic therapeutics, academic institutions and researchers will need to work closely and cooperatively with the industry to assure rapid drug development to benefit AD patients. Furthermore, regulatory and funding authorities will need to acknowledge the large unmet need and current lack of safe and adequate treatments for such a common disease in adults and children and support the efforts for translational and drug development for this disease. The recent advances in our understanding of the immunopathogenic mechanisms implicated in AD provide an opportunity for development of biological therapies directed at pathways driving AD. We believe that this is the beginning of a new and exciting era in AD therapeutics with impending availability of narrow-targeted drugs with low toxicity and increased patients’ compliance. These expected drugs will not only treat this disease, but also prevent the development and relapse of new skin lesions given the current therapeutic challenges of treating AD.
Acknowledgment
The Authors acknowledge Mr. SM Nawjes Ali, Laboratory Technologist-cum-Computer Operator, Medical Research Unit (MRU), MHWT, Uttara Model Town, Dhaka-1230 for composing the manuscript.
References
- Bieber T (2008) Atopic dermatitis. N Eng J Med 358(14): 1483-1494.
- Bieber T (2010) Atopic dermatitis. Ann Dermatol 22(2): 125-37.
- Williams H, Stewart A, Von Mutius E, Cookon W, Anderson HR (2008) International study of asthma and allergies in childhood (ISAAC) phase one and three study groups. Is eczema really on the increase worldwide? J Allergy Clin Immunol 121(4): 947-54.
- Lee JH, Kim EH, Cho J, Kim FIY, Suh I, et al. (2011) Comparison of prevalence and risk factors of atopic dermatitis by physical examination and questionnaire survey in elementary school children. Pediatr Allergy Respir Dis 21(3): 186-96.
- Ricci G, Patrizi A, Baldi E, Menna G, Tabanelli V, et al. (2006) Long term follow-up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol 55(5): 765-71.
- Guttman Yassky E, Nograles KE, Krueger JG (2011) Contrasting pathogenesis of atopic dermatitis and psoriasis-Part II: Immune cell subsets and therapeutic concepts. J Allergy Clin Immunol 127(6): 1420-32.
- Denby KS, Beck LA (2012) Update on systemic therapies for atopic dermatitis. Curr Opin Allergy Clin Immunol 12(4): 421-26.
- Novac N, Simon D (2011) Atopic dermatitis-from new pathophysiologic insights to individualized therapy. Allergy 66(7): 830-39.
- Barnes KC (2010) An update on the genetics of atopic dermatitis: scratching the surface in 2009. J Allergy Clin Immunol 125: 16-29 e1-11.
- Palmer CN, Irvine AD, Terron KA, Zhao Y, Liao H, et al. (2006) Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 38(4): 441- 41.
- Guttman Yassky E, Krueger JG, Lebwohl MG (2018) Systemic immune mechanisms in atopic dermatitis and psoriasis with implications treatment. Exp Dermatol 27(4): 409-417.
- Patel N, Strowd LC (2017) The future of atopic dermatitis treatment. Adv Exp Med Biol 1027: 185-210.
- Vasilopoulos Y, Cork MJ, Murphy R, Willams HC, Robinson DA, et al. (2004) Genetic association between an AACC insertion in the 3’UTR of the stratum chymotryptic enzyme gene and atopic dermatitis. J Invest Dermatol 123(1): 62-66.
- Morar N, Cookson WO, Harper JI, Moffatt MF (2007) Filaggrin mutations in children with severe atopic dermatitis. J Invest Dermatol 127(7): 1667-72.
- Peters EM, Raap U, Welker P, Tanaka A. Matsuda H, et al. (2007) Neuotropins act as neuroendocrine regulators of skin homeostasis in health and disease. Horm Metab Res 39(2): 110-24.
- Raap U, Kapp A (2005) Neuroimmunological findings in allergic skin diseases. Curr Opin Allergy Clin Immunol 5(5): 419-24.
- Proksch E, Folster-Holst R, Jensen JM (2006) Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci 43(3): 159-69.
- Rippke F, Schreiner V, Doering T, Maibach HI (2004) Stratum corneum pH in atopic dermatitis: impact on skin barrier function and colonization with Staphylococcus aureus. Am J Clin Dermatol 5(4): 217-23.
- Izadpanah A, Gallo RI (2005) Antimicribial peptides. J Am Acad Dermatol 52(3): 381-90.
- Girt LY, Beck IA (2006) Innate immune defects in atopic dermatitis. J Allergy Clin Immunol 118(1): 202-208.
- Rieg S, Steffen H, Seeber S, Humeny A, Kalbacher H, et al. (2005) Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol 174(12): 8003-10.
- Homey B, Steinhoff M, Ruzicka T, Leung DY (2006) Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol 118(1): 178-89.
- Beidermann T, Rocken M, Carballido JM (2004) TH1 and TH2 lymphocyte development and regulation of TH cell-mediated immune responses of the skin. J Investig Dermatol Symp Proc 9(1): 5-14.
- Fiset PO, Leung DY, Hamid Q (2006) Immunopathology of atopic dermatitis. J Allergy Clin Immunol 118(1): 287-90.
- Park JH, Choi YL, Namkung JH, Kim WS, Lee JH, et al. (2006) Characteristics of extrinsic vs intrinsic atopic dermatitis in infancy: correlations with laboratory variables. Br J Dermatol 155(4): 778-83.
- Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, et al. (2003) Polarised in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol 111(4): 875-81.
- Kim BE, Leung DY, Streib JE, Kisich K, Boguniewich M, et al. (2007) Macrophage inflammatory protein 3alpha deficiency in atopic dermatitis skin and role in innate immune response to vaccinia virus. J Allergy Clin Immunol 119(2): 457-63.
- Novak N, Bieber T (2005) The role of dendritic cell subtypes in the pathophysiology of atopic dermatitis. J Am Acad Dermatol 53: S171-176.
- Jung T, Sting G (2008) Atopic dermatitis: therapeutic concepts evolving from new pathophysiologic insights. J Allergy Clin Immunol 122(6): 1074-81.
- Kim BE, Leung DY (2012) Epidermal Barrier in Atopic Dermatitis. Allergy Asthma Immunol Res 4(1): 12-16.
- Guttman Yassky E, Dhingra N, Leung DYM (2013) New era of biological therapeutics in atopic dermatitis. Expert Opin Biol Ther Apr; 13(4): 549-61.
- Tintle S, Guttman E, Suárez-Fariñas M, Fujita H, Gilleaudeau P, et al. (2011) Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol 128(3): 583-93.
- Lee L, Soto D, Bielory L (2008) Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol 121(1): 116-21.
- Swamy RS, Reshamwala N, Hunter T, Vissamsetti S, Santos CB et al. (2012) Epigenetic modification and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol 130(1): 215-24.
- Souwer Y, Szegedi K, Kapsenberg ML (2010) IL-17 and IL-22 in atopic allergic disease. Curr Opinion Immunol 22(6): 821-26.
- Akkoc T, de Koning P, Ruckert B, Barlan I, Akdis M, et al. (2008) Increased activation-induced cell death of high IFN-[gamma]-producing THI cells as a mechanism of TH2 predominance in atopic disease. J Allergy Clin Immunol 121(3): 652-58.
- Navarini AA, French LE, Hofbauer GFL (2011) Interrupting IL-6-receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J Allergy Clin Immunol 128(5): 1128-30.
- Buka RL, Resh B, Roberts B, Cunningham BB, Friedlander S (2005) Etanercept is minimally effective in 2 children with atopic dermatitis. J Am Acad Dermatol 53(2): 358-59.
- Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, et al. (2008) Adminstration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol 122: 829-31.
- Heimall J, Spergel JM (2012) Filaggrin mutations and atopy: consequences for future therapeutics. Expert Rev Clin Immunol 8(2): 189-97.
- Grey K, Maguiness S (2016) Atopic Dermatitis: Update Pediatricians. Pediatr Ann. 45(8): e280-6.
- D Auria E, Banderali G, Barberi S, Gualandri L, Pietra B, et al. (2016) Atopic dermatitis : recent insight on pathogenesis and novel therapeutic target. Asian Pac J Allergy Immunol 34(2): 98-108.
- Kim MJ, Kim SM, Lee YW, Choe YB, Ahn KJ (2016) Vitamin D status and efficacy of Vitamin D supplementation in atopic dermatitis: A systematic review and meta-analysis. Nutrients 8(12): pii-E789.
- Bussmann C, Bockenhoff A, Henke H, Werfel T, Novak N (2006) Does allergen- specific immunotherapy represent a therapeutic option for patients with atopic dermatitis? J Allergy Clin Immunol 118(6): 1292-98.
- Compalati E, Rogkakou A, Passalacqua G, Canonica GW (2012) Evidences of efficacy of allergen immunotherapy in atopic dermatitis: an updated review. Curr Opin Allergy Clin Immunol 12(4): 427-33.
- Werfel T, Breuer K, Rueff F, Przybilla B, Worm M, et al. (2006) Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy 61(2): 202-205.
- Landolina N, Levi-Schaffer F (2016) Monoclonal antibodies: the new magic bullets for allergy: IUPHAR Review 17. Br J pharmacol 173(5): 793-803.
- Sediva A, Kayserova J, Vernerova E, Poloucková A, Capková S, et al. (2008) Anti-CD20 (rituximab) treatment for atopic eczema. J Allergy Clin Immunol 121(6): 1515-16.
- Simon D, Hosli S, Kostylina G, Yawalkar N, Simon HU (2008) Anti-CD20 (rituximab) treatment improves atopic eczema. J Allergy Clin Immunol 121: 122-28.
- Namkung JH, Lee JE, Kim E, Kim HJ, Seo EY et al. (2011) Association of polymorphisms in genes encoding IL-4, IL-13 and their receptors with atopic dermatitis in a Korean population. Exp Dermatol 20(11): 915-19.
- Roesner LM, Werfel T, Heratizadeh A (2016) The adaptive immune system in atopic dermatitis and implications on therapy. Expert Rev Clin Immunol 12(7): 787-96.
- Buzney CD, Gottlieb AB, Rosmarin D (2016) Asthma and atopic dermatitis: A review of targeted inhibition of Interleukin-4 and Interleukin-13 as therapy for atopic disease. J Drugs Dermatol15(2): 165-171.
- Molfino NA, Gossage D, Kolbeck R (2012) Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy 42(5): 712-737.
- Oldhoff JM, Darsow U, Werfel T (2006) No effect of anti-interleukin-5 therapy (mepolizumab) on the atopy patch test in atopic dermatitis patients. Int Arch Allergy Immunol 141(3): 290-294.
- Cornelissen C, Marquardt Y, Czaja K (2012) IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol 129(2): 426-433.
- Cheung PF, Wong CK, Ho AW (2010) Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol 22(6): 453-467.
- Heratizadeh A, Werfel T (2016) Anti-inflammatory therapies in atopic dermatitis 71(12): 1666-1675.
- Nograles KE, Zaba LC, Shemer A (2009) IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 123(6): 1244- 1252.
- Souwer Y, Szegedi K, Kapsenberg ML (2011) IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol 22(6): 821-826.
- Milner JD (2011) IL-17 producing cells in host defense and atopy. Curr Opin Immunol 23(6): 784-788.
- Aral M, Arican O, Gul M (2006) The relationship between serum levels of total IgE, IL-18, IL-12, IFN-gamma and disease severity in children with atopic dermatitis. Mediators Inflamm 2006(4): 73098.
- Jacobi A, Antoni C, Manger B (2005) Infliximab in the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol 52(3): 522-526.
- Behshad R, Cooper KD, Korman NJ (2008) A retrospective case series review of the peroxisome proliferator-activated receptor ligand rosiglitazone in the treatment of atopic dermatitis. Arch Dermatol 144(1): 84-88.
- Ogata A, Fujieda Y, Terakawa M (2011) Pharmacokinetic/pharmacodynamic analyses of chymase inhibitor SUN13834 in NC/Nga mice and prediction of effective dosage for atopic dermatitis patients. Int Immunopharmacol 11(10): 1628-1632.
© 2019 ASM Giasuddin. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






