- Submissions

Full Text
Global Journal of Endocrinological Metabolism
A Randomized, Double-Blind, Placebo-Controlled Trial to Assess the Efficacy and Safety of Hydroxychloroquine in Patients with Type 2 Diabetes Mellitus
Davis Townsend*, Michaela Eynatten and Jody Norton
Department of Endocrinology, University of Minami, USA
*Corresponding author: Davis Townsend, Department of Endocrinology, University of Minami, 1600 NW 10th Ave, Miami, FL 33136, USA
Submission: July 30, 2018;Published: August 14, 2018

ISSN: 2637-8019Volume2 Issue4
Abstract:
Objective: Observational study indicate that Hydroxychloroquine (HCQ) 400mg helps to achieve target glycemic parameter as add on in inadequately control type 2 diabetes patients (T2DM). We examine the effect of Hydroxychloroquine on reduction in glycemic parameters in uncontrolled T2DM patients.
Methods: A 6month randomized, double-blind, placebo controlled trial of hydroxychloroquine and placebo in 39subjects (22m/17f) of ages 40-70years (y), with HbA1c >8%, Weight >60kg, and T2DM 12 months was conducted at a university outpatient facility. The hydroxychloroquine group consisted of 20 subjects (12m/8f), of age 55.0±2.5 y; while the control group was made up of 19 subjects (10m/9f), of age 54.5±3.1y. Patients were randomized to double-blind hydroxychloroquine 400mg OD or placebo added on to metformin 1500mg and Glimepiride 4mg following a 4 week. The primary end point was the change from baseline to week 12 in HbA1c. Key secondary end points included change from baseline to week 24 in fasting plasma glucose (FPG), post prandial plasma glucose (PPG), change in weight and the proportion of patients achieving HbA1c target (< 7%).
Result: There was a significantly greater reduction in HbA1c at 24 weeks with HCQ add-on (-1.3±0.5% [p=0.001]) versus placebo (-0.6±0.01%[p=0.031]). There was statistically significant reductions in fasting plasma glucose and 2-h postprandial glucose with HCQ group as compared to placebo group. A larger proportion of patients achieved HbA1c < 7% with HCQ add-on (60%) versus placebo add-on (21%). Adverse events were similar between treatment groups. Episodes of hypoglycaemia were infrequent in both treatment arms, and there were no episodes of major hypoglycaemia. There were statistically reductions in mean body weight (LOCF) in HCQ group. Mean change in body weight (95% CI) at week 24 was 6.12±1.1kg for the HCQ group and increased +0.9±0.4 kg for the placebo group.
Conclusion: Patients with inadequately controlled type 2 diabetes mellitus, HCQ 400mg provided clinically meaningful improvements in glycemic control without weight gain or increased risk of hypoglycaemia.
Keywords: Hydroxychloroquine; Type 2 diabetes mellitus; HbA1c
Introduction
Type 2 diabetes is a progressive disease and multiple factors were contributing towards its complication [1]. Only with lifestyle interventions or medication therapy with a single agent, adequate glycemic control may not be possible. American Diabetes Association [2] and other guidelines [3] recommend combination therapy when glycated haemoglobin (HbA1c) goal < 7% is not achieved or maintained over a 3 to 6 month period. Hydroxychloroquine inhibits insulin degrading enzyme by changing pH of cellular media and therefore may partially increase intercellular insulin availability. Considering the multifaceted effects of hydroxychloroquine, it could slow down the progression from the pre-diabetes stage to diabetes and can also improve the cardiovascular risk profile in diabetes patients with its favourable actions on blood glucose, lipid profile and antithrombotic properties, making it an attractive add on therapeutic choice for the treatment of T2DM patients. There are few randomised trial as well as few observational study indicate that Hydroxychloroquine (HCQ) 400 mg helps to achieve target glycemic parameter as add on in inadequately control type 2 diabetes patients(T2DM). For example, a 24-week trial in 267 patients demonstrated significant reductions in HbA1c and fasting plasma glucose (FPG) with hydroxychloroquine 400mg as compare to pioglitazone 15mg [4]. The change from baseline in HbA1c at 24 weeks was similar between the 2 treatment groups.
A 24 week trial [5] in 240 patients revealed significantly greater reductions in insulin dose and HbA1c by 1.3% form baseline with hydroxychloroquine 400 mg poorly controlled type 2 diabetes on stable insulin therapy along with glimepiride and metformin. ARCT study conducted by Quatraro et al. [6], there is a reduction in insulin dose by an average of 30% when hydroxychloroquine used along with insulin. We examine the effect of Hydroxychloroquine on reduction in glycemic parameters in uncontrolled T2DM patients.
Methods
A 6month randomized, double-blind, placebo controlled trial of hydroxychloroquine and placebo in 39subjects (22m/17f) of ages 40-70years (y), with HbA1c >8%, Weight >60Kg, and T2DM 12months was conducted at a university outpatient facility. The hydroxychloroquine group consisted of 20 subjects (12m/8f), of age 55.0±2.5 y; while the control group was made up of 19 subjects (10m/9f), of age 54.5±3.1y. At the screening visit, each patient was assigned a unique sequential subject number by an Interactive Voice Response System (IVRS), which was used for identification throughout the study. Patients were randomized 1:1 to double-blind hydroxychloroquine 400mg OD or placebo added on to metformin 1500mg and Glimepiride 4 mg following a 4 week. The computer-generated randomization scheme was developed and kept by the investigator. Randomization was performed by calling the IVRS. Placebo tablets were identical in appearance to the hydroxychloroquine tablets, and medication was dispensed using bottle numbers assigned by the IVRS. Titration or adjustment of blinded hydroxychloroquine or metformin was not allowed during the study. The primary end point was the change from baseline to week 12 in HbA1c. Key secondary end points included change from baseline to week 24 in fasting plasma glucose (FPG), post prandial plasma glucose (PPG), change in weight and the proportion of patients achieving HbA1c target (< 7%).
The main Inclusion criteria was patients with Type 2 diabetes mellitus (T2DM) with inadequate glycemic control, defined as a central laboratory glycosylated haemoglobin (HbA1c) ≥7.5% and 9% obtained at the screening visit and weight ≥60kg. The main exclusion criteria existed any cardiovascular vascular diseases prior to screening and any type of retinal abnormalities. The protocol, amendments, and patient informed consent were approved by the institutional review board (IRB)/independent ethics committee (IEC) at each site before study initiation, and the study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonisation/Good Clinical Practice. Patients provided informed consent before study participation. Each IRB/IEC was composed of a review panel that was responsible for ensuring the protection of the rights, safety, and well-being of human subjects involved in the clinical investigation and was adequately constituted to provide assurance of that protection.
Baseline and change from baseline efficacy assessments were analysed in the Randomized Patients Population (those who received randomized study drug with ≥1 post base line assessment). The primary efficacy analysis was an analysis of covariance (ANCOVA) of the adjusted mean change in HbA1c (leastsquares mean adjusted for baseline HbA1c value) from baseline to week 24 (or LOCF) during the double-blind period with treatment as a fixed effect and baseline HbA1c as a covariate. Change from baseline to week 24 in FPG was analysed in the same way as the primary end point. The number and proportion of patients achieving a therapeutic glycemic response (HbA1c < 7.0%) at week 24 LOCF was compared between groups.
Result
Patients demographic and baseline characteristics were described in details at Table 1. The hydroxychloroquine group consisted of 20 subjects (12m/8f), of age 55.0±2.5 y; while the control group was made up of 19 subjects (10m/9f), of age 54.5±3.1y. At week 24, adjusted mean reductions (95% CI) from baseline in HbA1c were significantly greater (P = 0.001) in the HCQ group (-1.3±0.5%) versus the placebo group (-0.6±0.01%) (Table 2). At week 24, adjusted mean reductions from baseline in the first secondary end point, FPG, were significantly greater (-60±16.7mg/ dl) in the HCQ group than in the placebo group (-24.9±13.4 mg/ dl), and the difference (95% CI) in between groups at week 24 was statistically significant (P = 0.001) (Table 2). At week 24 the adjusted mean reductions from baseline in PPG were also significantly greater (-135±19.9 mg/dl) in the HCQ group than in the placebo group (-42±19.2mg/dl) and the difference (95% CI) in between groups at week 24 was statistically significant (P = 0.001).
Table 1:Patient demographic and base line characteristics (randomized patient’s population).
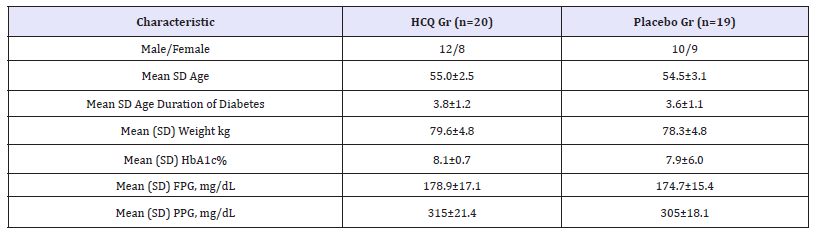
Table 2:Primary key secondary end points (randomised patient’s population).

The percentage of patients achieving a therapeutic glycemic response HbA1c < 7%) was numerically greater with HCQ group versus placebo group (60% [12/20] vs. 21% [4/19]). The difference (95% CI) in the proportions of patients achieving HbA1c< 7% in HCQ group versus placebo was statistically significant (P = 0.001). Few patients in either group discontinued the study because of lack of glycemic control at week 4 (Placebo group, n=1).
There were statistically reductions in mean body weight (LOCF) in HCQ group where there was a weight increase in placebo group. Mean change in body weight (95% CI) at week 24 was -6.12±1.1kg for the HCQ group and +0.9±0.4 kg for the placebo group.
Discussion
In the study reported here, HCQ 400mg OD in combination with metformin and glimepiride for 24 weeks significantly reduced HbA1c in patients with type 2 diabetes with inadequate glycemic control. Reduction in FPG, PPG and HbA1c was observed relative to corresponding baseline values in both the groups. In the United States, only 52% of adults with diagnosed diabetes have HbA1c < 7.0% [5]. Thus there is a need for new therapeutic approaches that get more patients to their individual glycemic goal without increased risk of hypoglycemia or weight gain. The proportions of patients achieving HbA1c < 7% were numerically greater for the HCQ group compared with the placebo group and consistent with previous short-term studies [7] of HCQ added to existing oral antihyperglycemic therapy.
In patients with type 2 diabetes, hypoglycemia is associated with increased morbidity and mortality [8], whereas body weight reduction is associated with improved glycemic control and a reduction in cardiovascular risk factors [9,10]. Thus hypoglycemic potential and effects on body weight are important attributes to be considered when choosing a drug as an add-on to existing therapy, as highlighted in clinical treatment guidelines. In this trial, HCQ were associated with low rate of hypoglycemia when used with metformin and sulfonylurea and it significantly reduce the body weight along with tight glycemic control.
Conclusion
Patients with inadequately controlled type 2 diabetes mellitus, hydroxychloroquine provided clinically meaningful improvements in glycemic control without weight gain or increased risk of hypoglycemia.
Disclosure
No funding for this review. Davis Townsend has served as a consultant and/or member of an advisory board or speaker’s bureau for Sanofi-Aventis, Astra Zeneca, Bristol-Myers Squibb, Eli Lilly and Novartis.
References
- Zinman B (2011) Initial combination therapy for type 2 diabetes mellitus: Is itready for prime time? Am J Med 124(1 Suppl): S19-S34.
- Professional Practice Committee (2018) Standards of medical care in diabetes-2018. Diabetes Care 41(Supplement 1): S1-S3.
- Alan JG, Martin JA, Joshua IB, Lawrence B, Zachary TB, et al. (2018) AACE/ACE consensus statement. Endocrine Practice 24(1): 1-30.
- Pareek A, Chandurkar N, Thomas N, Viswanathan V, Deshpande A, et al. (2014) Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: A double blind, randomized comparison with pioglitazone. Curr Med Res Opin 30(7): 1257-1266.
- Baidya A, Chakravarti HN, Saraogi RK, Gupta A, Ahmed R, et al. (2018) Efficacy of maximum and optimum doses of hydroxychloroquine added to patients with poorly controlled type 2 diabetes on stable insulin therapy along with glimepiride and metformin: association of high sensitive c-reactive protein (Hs-CRP) and glycosylated haemoglobin (HbA1c). Endocrinol Metab Syndr 7: 283.
- Quatraro A, Consoli G, Magno M, Caretta F, Nardozza A, et al. (1990) Hydroxychloroquine in decompensated, treatment-refractory noninsulin dependent diabetes mellitus. A new job for an old drug? Ann Intern Med 112(9): 678-681.
- Jagnani VK, Bhattacharya NR, Satpathy SC, Hasda GC, Chakraborty S (2017) Effect of hydroxychloroquine on type 2 diabetes mellitus unresponsive to more than two oral anti-diabetic agents. J Diabetes Metab 8: 771.
- Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, et al. (2013) Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 368(17): 1613-1624.
- Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, et al. (2011) Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 34(5): 1164-1170.
- Horton ES, Silberman C, Davis KL, Berria R (2010) Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care 33(8): 1759-1765.
© 2018 Davis Townsend,. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






