- Submissions

Full Text
Gerontology & Geriatrics Studies
Epileptogenesis and/in Dementia
Mirko Avesani* and Alfonso Ciccone
Department of Neurological Sciences, Division of Neurology, Italy
*Corresponding author: Mirko Avesani, Department of Neurological Sciences, Division of Neurology, Italy
Submission: June 06, 2020; Published: September 23, 2021

ISSN 2578-0093Volume7 Issue2
Abstract
In this review, we analyze the possible linkage between epilepsy and dementia, considering the actual knowledges about epileptogenesis. In the final part, we also speak about the treatment of epilepsy in dementia and of dementia in epilepsy.
Introduction
With advances in healthcare and an ageing population, the number of older adults with epilepsy is set to rise substantially across the world. In developed countries the highest incidence of epilepsy is already in people over 65 and, as life expectancy increases, individuals who developed epilepsy at a young age are also living longer. Recent findings show that older persons with epilepsy are more likely to suffer from cognitive dysfunction and that there might be an important bidirectional relationship between epilepsy and dementia.
Thus, some people with epilepsy may be at a higher risk of developing dementia, while individuals with some forms of dementia, particularly Alzheimer’s disease and vascular dementia, are at significantly higher risk of developing epilepsy [1].
Epilepsy and Dementia
Figure 1:
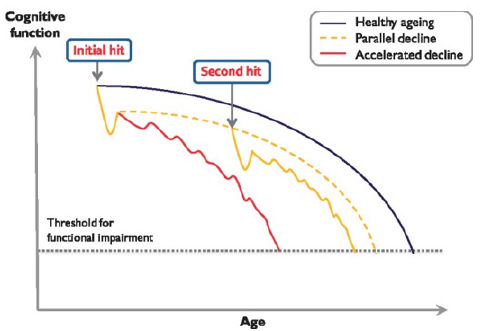
Whether epilepsy simply lowers brain reserve and thereby facilitates manifestation of dementia-related pathology, or whether epilepsy is a disorder that itself produces dementia-by the effects of seizures and IEDs on brain structure and function also remains unclear. The evidence certainly suggests that some vascular risk factors and pathophysiological mechanisms
(e.g., tau and vascular pathology) are indeed common to people with epilepsy and dementia. Another important future direction of research would be to investigate whether more aggressive management of vascular risk factors (e.g., blood pressure, smoking, diet and exercise) would improve control of seizures and thereby protect against worsening of cognitive decline in people with epilepsy. Likewise, an important related, unanswered question is whether elderly patients with epilepsy are genuinely susceptible to accelerated cognitive decline (Figure 1).Does the pathological burden (vascular, traumatic, tau or amyloid) in people with epilepsy interact synergistically with ongoing seizure activity or IEDs to lead to progressive worsening of cognitive function and further deviation away from the normal trajectory associated with cognitive ageing? Longitudinal studies of older patients with epilepsy could potentially assist greatly in this regard [1].
In clinical practice, seizures in patients with Alzheimer’s disease can easily go unrecognized because they usually present as non-motor seizures and can overlap with other symptoms of the disease. In patients with Alzheimer’s disease, seizures can hasten cognitive decline, highlighting the clinical relevance of early recognition and treatment. Some evidence indicates that subclinical epileptiform activity in patients with Alzheimer’s disease, detected by extended neurophysiological monitoring, can also lead to accelerated cognitive decline. Treatment of clinical seizures in patients with Alzheimer’s disease with select Antiepileptic Drugs (AEDs), in low doses, is usually well tolerated and efficacious. Moreover, studies in mouse models of Alzheimer’s disease suggest that certain classes of AEDs that reduce network hyperexcitability have disease-modifying properties. These AEDs target mechanisms of epileptogenesis involving amyloid β and tau. Clinical trials targeting network hyperexcitability in patients with Alzheimer’s disease will identify whether AEDs or related strategies could improve their cognitive symptoms or slow decline [2].
It has been proposed that inflammatory reactions occurring in various CNS diseases, can, as a risk factor, predispose to dementia. Inflammatory reactions occur in the brain in various CNS diseases, including autoimmune, neurodegenerative, and epileptic disorders. Proinflammatory and anti-inflammatory cytokines and related molecules have been described in CNS and plasma, in experimental models of seizures and in clinical cases of epilepsy. Inflammation involves both the innate and the adaptive immune systems and shares molecules and pathways also activated by systemic infection. Experimental studies in rodents show that inflammatory reactions in the brain can enhance neuronal excitability, impair cell survival, and increase the permeability of the blood-brain barrier to bloodborne molecules and cells. Moreover, some anti-inflammatory treatments reduce seizures in experimental models and, in some instances, in clinical cases of epilepsy. However, inflammatory reactions in brain also can be beneficial, depending on the tissue microenvironment, the inflammatory mediators produced in injured tissue, the functional status of the target cells, and the length of time the tissue is exposed to inflammation [3].
Pathogenesis
Cognitive comorbidities are very common in epilepsy and often seen as secondary to epilepsy or caused by epilepsy. The implicit and sometimes explicit assumption is that epilepsy damages the brain and thus leads to functional deterioration and behavioral alterations. The central is whether there is a bidirectional relationship between epilepsy and cognition. In this regard it is essential to disentangle what is the disease and what is the symptom. Cognitive problems often exist from the onset of epilepsy, if not before, and the impact of epilepsy on cognition cannot be discerned without also considering the underlying brain pathology and its dynamics. Unraveling the etiologies of epilepsy increasingly reveals conditions wherein epilepsy, cognitive and behavioral problems are all symptoms of a common underlying pathological condition. Functional reserve capacities determine the outcome of epilepsy and its treatment. A functional interrelationship exists between epilepsy and behavior, since epileptic activity can affect behavior and behavior can alter epileptic activity. In conclusion, an epilepsy-centric unidirectional view of the behavioral problems being caused by epilepsy is obsolete. Such a view may even prevent the search for and treatment of the underlying etiological factors. Instead, a practical clinical approach is favored according to which the comorbidities of epilepsy must be diagnosed at the onset of the disease, and according to which comorbidities may require separate treatment approaches [4].
Epilepsy and dementia in older patients: a bidirectional relationship?
Gowers [5] first introduced the concept of epileptic dementia, implying that dementia and epilepsy might, in some subjects, be the consequence of the same underlying disorder [5]. The higher incidence of cognitive impairment in older people with epilepsy certainly raises questions as to whether these individuals might have increased rates of progression to dementia, particularly those with TLE [6]. As part of the EURODEM project, eight case-control studies that assessed the risks of developing Alzheimer’s disease in several medical conditions were reanalysed [7]. When compared with population-based controls, individuals with epilepsy had an increased relative risk of being diagnosed with Alzheimer’s disease at least 1 year after epilepsy diagnosis. The greatest risk for Alzheimer’s disease occurred in patients who had epilepsy for <10 years (relative risk 2.5) versus >10 years (relative risk 1.4). Importantly though, this increased risk appeared not to be related to the cumulative effect of longstanding seizures. A follow-up study based on three nationwide Dutch morbidity registers over the period 1980-89 investigated the risk of dementia for patients aged 50-75 years. Patients with epilepsy were found to have a relative risk of 1.5 of being diagnosed with dementia over an 8-year period [8].
The mechanisms underlying an increased likelihood of developing dementia have not been definitively established. Intriguingly, part of the risk might be attributable to the fact that patients with Alzheimer’s disease and/or vascular dementia are actually more likely to develop epilepsy [9,10]. In a UK study that examined the records of 22 084 patients (mean age ∼80 and half the sample with Alzheimer’s disease), after adjusting for baseline predictors of seizures, Alzheimer’s disease was associated with a significantly increased risk of seizures [Hazard Ratio (HR) 5.31, 95% Confidence Interval (CI) 3.97-7.10] [11]. In addition, patients with Alzheimer’s disease were at a higher risk of haemorrhagic stroke (HR 1.49, 95% CI 1.06-2.08), which is an independent risk factor for developing seizures. In Han Chinese patients with Alzheimer’s disease too, the risk of seizures is higher in Alzheimer’s disease than in age-matched controls (HR 1.85; 95% CI 1.40-2.90) [12].
Vascular dementia also increases the risk of seizures (Figure 2). Another large UK population-based study examined the records of 4438 cases with vascular dementia and 7086 patients with Alzheimer’s disease, comparing them to 11 524 dementiafree patients (mean age >80 in the three samples). This reported an odds ratio of developing seizures of 5.7 (95% CI 3.2-10.1) for vascular dementia and 6.6 (95% CI 4.1-10.6) for Alzheimer’s disease [10]. Thus, it might not simply be a case of epilepsy and associated risk factors, increasing the risk of dementia, but dementia-whether in the form of Alzheimer’s disease or vascular dementia-simultaneously increasing the risk of epilepsy [4].
Figure 2:Comorbidities in old patient.
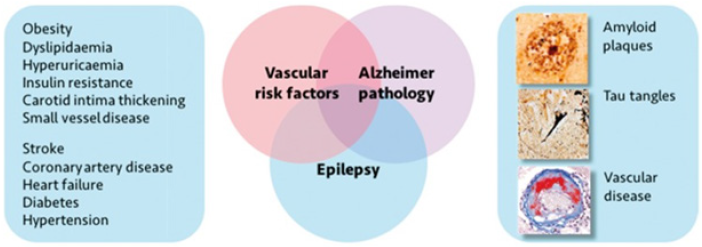
These considerations raise important questions: do seizures cause dementia, does dementia cause seizures, or does something else cause both? Some recent work suggests that epilepsy could be considered a symptom rather than a disease in itself [4]. It is argued that epilepsy is simply one manifestation of the underlying pathological process that might contribute to seizures, cognitive decline, psychological problems, systemic illness and, perhaps indirectly, psychosocial difficulties. Seizures can, for example, be observed in the prodromal phase of several neurodegenerative illnesses [13], and some studies in patients with mild cognitive impairment or Alzheimer’s disease have reported that cognitive decline may begin several years earlier in those individuals who suffer from seizures compared to those who do not [14-16]. These findings have led some authors to consider whether there might be analogous processes occurring in patients with TLE who might progress to dementia [6].
In patients with familial autosomal dominant early-onset Alzheimer’s disease (<50 years old) seizures are far more common than in typical late-onset Alzheimer’s disease, affecting >45% of cases, although rates vary with specific genetic mutations [17]. This suggests that younger people with Alzheimer’s disease (aged 50- 59) are at highest risk of developing seizures and therefore disease duration might not be crucial [14]. There is also some evidence that epilepsy in typical late-onset Alzheimer’s disease may be associated with faster progression of cognitive decline [14,18], but large-scale longitudinal studies are required to confirm this. As highlighted in a comprehensive recent systematic review, much more work is needed to fully elucidate the epidemiology of epilepsy in dementia and also of dementia in epilepsy [19]. It remains unclear whether epilepsy and dementia simply share common risk factors, whether there is truly a bidirectional relationship between the two-or both. In the next section we examine evidence for common risk factors.
Network hyperexcitability in Alzheimer’s disease
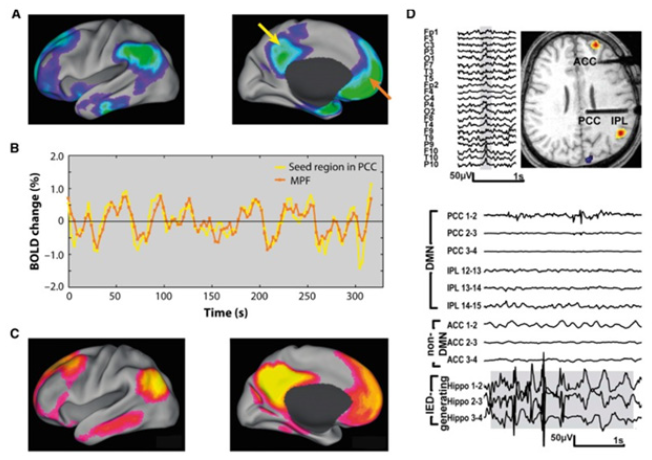
In patients with mild cognitive impairment due to Alzheimer’s disease, task-based fMRI studies have shown increased hippocampal activation during memory encoding [20]. Although this activation can be interpreted as the compensatory recruitment of neural reserves to meet cognitive demands within compromised brain regions, some evidence indicates that hyper activation of the hippocampus and multiple other cortical and subcortical brain regions during encoding is inefficient and might contribute to Alzheimer’s disease pathogenesis [20,21]. The amount of hippocampal hyper activation in patients with mild cognitive impairment due to Alzheimer’s disease might predict future cognitive decline [20]. Furthermore, in patients with amnestic mild cognitive impairment, suppressing hippocampal dentate gyrus and CA3 hyperactivation with levetiracetam improves their performance on a hippocampal-based pattern-separation task [22]. Interestingly, 125mg twice a day of levetiracetam was more effective than 250mg twice a day at normalizing fMRI changes and improving memory function in these patients [22]. Since fMRI is an indirect measure of neuronal activity, the association between hippocampal hyperactivation detected by fMRI and epileptiform activity (detected by EEG) is poorly understood.
Regions outside the hippocampus also show excess network activity and synchrony in early stages of Alzheimer’s disease. For instance, patients with mild cognitive impairment or mild Alzheimer’s disease, and cognitively healthy people with PET evidence for amyloid β deposition have aberrant activity in the default network, including the precuneus and posterior cingulate cortex, indicated by failure to deactivate this network during memory encoding [23]. Additionally, resting-state MEG has shown network hypersynchronisation across multiple frequency bands in frontoparietal and interhemispheric networks in patients with amnestic mild cognitive impairment [24].
We propose that a crucial period during mild cognitive impairment stages exists, in which hippocampal hyperactivation (Figure 3) is associated with an increase in deposition of neurofibrillary tangles while amyloid β deposition begins to plateau [20,25]. Brain deposits of amyloid β and tau are not as tightly linked with cognitive dysfunction in human beings as soluble forms of these proteins are in animal models of Alzheimer’s disease [26]. Since non-invasive means of measuring soluble protein in the brain are not available, functional imaging and neurophysiological recordings might become crucial biomarkers of the functional effects of these proteins, both for protein-lowering approaches and other network-stabilizing therapeutic strategies.
Figure 3:
Dementia after years of focal epilepsy: the case of Temporal Lobe Epilepsy
The association of epilepsy with structural brain changes and cognitive abnormalities in midlife has raised concern regarding the possibility of future accelerated brain and cognitive aging and increased risk of later life neurocognitive disorders. To address this issue a study (Hwang, 2020), recently examined age-related processes in both structural and functional neuroimaging among individuals with Temporal Lobe Epilepsy (TLE, N=104) who were participants in the Epilepsy Connectome Project (ECP). Support Vector Regression (SVR) models were trained from 151 healthy controls and used to predict TLE patients’ brain ages. It was found that TLE patients on average have both older structural (+6.6 years) and functional (+8.3 years) brain ages compared to healthy controls. Accelerated functional brain age (functional-chronological age) was mildly correlated (corrected P=0.07) with complex partial seizure frequency and the number of anti-epileptic drug intake. Functional brain age was a significant correlate of declining cognition (fluid abilities) and partially mediated chronological age-fluid cognition relationships. Chronological age was the only positive predictor of crystallized cognition. Accelerated aging is evident not only in the structural brains of patients with TLE, but also in their functional brains. Understanding the causes of accelerated brain aging in TLE will be clinically important in order to potentially prevent or mitigate their cognitive deficits.
Treatment approaches of epilepsy with dementia and of dementia with epilepsy
Several factors must be considered when deciding to treat seizures in patients with Alzheimer’s disease, including age, comorbidities, drug interactions, cognitive and non-cognitive sideeffects, and optimal dose. In patients with epileptiform activity but no witnessed seizures, the decision of whether to treat with AEDs is controversial and should be based on the clinician’s judgment on whether so-called silent seizures might be contributing to cognitive symptoms (eg, cognitive fluctuations or confusion on waking). Empirical treatment with AEDs is not recommended for patients without clinical or electro graphical signs of network hyperexcitability. Such decisions must be guided by future clinical trials targeting network hyperexcitability in Alzheimer’s disease. The exact mechanism of action of most AEDs is unknown, and mechanisms might differ depending on dose. Because of the absence of randomised clinical trials addressing Alzheimer’s disease-associated seizures, therapeutic choices have to be based on evidence from other studies of AEDs in older adults with or without dementia [2].
The use of levetiracetam and lamotrigine to treat seizures associated with Alzheimer’s disease is supported by the strongest evidence. Notably, both AEDs can reduce excessive glutamate release from excitatory neurons, which might be relevant to epileptogenesis in Alzheimer’s disease [27,28]. Belcastro et al. [29] did an open-label, observational study of levetiracetam (1000- 1500mg per day) treatment for 1 year or longer in 25 patients with advanced Alzheimer’s disease and epilepsy [29]. 16% of participants discontinued the medication because of intolerable side-effects, and 72% were seizure-free for at least 1 year. Cumbo et al. [30] did a randomised, three-group parallel case-control study of levetiracetam (500-2000mg per day), lamotrigine (25-100mg per day), and phenobarbital (50-100mg per day) in 95 patients with Alzheimer’s disease and epilepsy (mean age 72 years) and 68 age-matched control patients with Alzheimer’s disease but without epilepsy, who did not receive AEDs [30]. Treatment consisted of a 4-week dose adjustment period followed by a 12-month dose evaluation period. Levetiracetam and lamotrigine caused fewer adverse events than phenobarbital, which caused somnolence in 30% of patients. 17% of patients on phenobarbital withdrew because of adverse effects. The three AEDs had equivalent effects on seizure reduction (response rates: levetiracetam 71%, lamotrigine 59%, and phenobarbital 64%). Levetiracetam and lamotrigine treatment resulted in better cognitive outcomes than phenobarbital, and lamotrigine improved mood. Patients with both Alzheimer’s disease and epilepsy who took levetiracetam had improved performance on MMSE and Alzheimer’s disease Assessment Scale-cognitive (ADAS-cog), similar to patients with Alzheimer’s disease and without epilepsy. A small, double-blind, crossover trial of lamotrigine (150 or 300mg per day) for patients with Alzheimer’s disease without epilepsy showed that treatment with 300 mg of lamotrigine was associated with improved performance on recognition and naming tasks as well as depressed mood on the ADAS behavior subscale after 8 weeks of treatment [31].
In the Veterans Administration Cooperative Study, Rowan et al. [32] did a randomised, double-blind, parallel trial comparing the tolerability and efficacy of gabapentin (1500mg per day), lamotrigine (150mg per day), and carbamazepine (600mg per day) for 12 months in a cohort of 593 adults (mean age 72 years) with new-onset epilepsy [32]. Notably, 66% of participants had vascular disease based on the prevalence of stroke, cardiac disease, and hypertension, while 35% of participants had mild cognitive impairment. Overall, patients tolerated lamotrigine and gabapentin better than carbamazepine. Seizure control was similar with all three AEDs, and more than half of participants remained seizure free at 12 months.
A multicenter, randomized, double-blind, placebo-controlled trial assessed the efficacy of valproic acid (10-12mg/kg per day), given over 2 years, to treat agitation in 313 patients with moderate Alzheimer’s disease without epilepsy [33,34]. Valproic acid treatment did not reduce incidence of agitation or psychosis, and was associated with higher rates of somnolence, gait disturbance, tremor, diarrhoea, and muscle weakness. Patients who were taking valproic acid also showed greater brain volume loss and faster decline in MMSE at 1 year than patients in the placebo group. These results should dissuade use of valproic acid, at least at the doses used in this trial, as a first-line therapy for patients with Alzheimer’s disease with or without epilepsy.
Evidence for using phenytoin in patients with Alzheimer’s disease is mostly limited to observational studies that have reported variability in its efficacy in seizure control and neurological side-effects, including ataxia, delirium, sedation, and accelerated cognitive decline [16,35,36]. Phenytoin paradoxically exacerbates seizures in the APP-J20 mouse model [37], but not in mice overexpressing APP and PS1 [38]. The deleterious effects of phenytoin on seizures and cognition in hAPP-J20 mice might be related to the depletion of Nav1.1 in parvalbumin-positive interneurons in the parietal cortex, a change also observed in brain samples from patients with Alzheimer’s disease [37]. Blocking Nav1.1 activity with phenytoin might exacerbate cortical excitability by reducing activity of Nav1.1-containing interneurons and disinhibiting neighboring pyramidal neurons. Lamotrigine also inhibits sodium channels but has a higher potency for inhibiting release of glutamate than of GABA, which distinguishes it from phenytoin [27]. Individuals with Down’s syndrome who develop epilepsy early in life usually tolerate phenytoin well, but those who develop Alzheimer’s disease and epilepsy later in life can have deleterious cognitive side-effects when taking phenytoin [35].
Benzodiazepines should be used as a last resort to treat seizures associated with Alzheimer’s disease. Although these drugs are highly effective at suppressing seizures and myoclonus, they can induce delirium and their sudden discontinuation (eg, when forgetting to take the medication) can induce withdrawal seizures, even in patients without epilepsy. Furthermore, chronic benzodiazepine use in an older population has been associated with increased risk of developing Alzheimer’s disease [38]. Topiramate reduces behavioural deficits in mouse models of Alzheimer’s disease [39]; however, its cognitive side-effects, such as word-finding difficulty and reduced concentration span, make it less appealing as a treatment for patients with Alzheimer’s disease. Other AEDs, such as oxcarbazepine and lacosamide, are being used successfully as monotherapy to treat seizures in older adults, but limited data are available for their use in patients with Alzheimer’s disease. Patients on carbamazepine or oxcarbazepine should have sodium levels monitored regularly, as these AEDs can cause hyponatraemia, particularly with advanced age.
Some symptomatic treatments for Alzheimer’s disease have been associated with an increased risk of seizures. In particular, the antidepressant bupropion and typical neuroleptics can decrease the seizure threshold in these patients. Acetylcholinesterase inhibitors, the mainstay of current symptomatic treatments, are likely to have neutral effects on seizure risk. A randomised, double-blind, placebo-controlled study of donepezil in patients with epilepsy did not reveal an increased frequency of seizures [40]. Memantine, a non-competitive NMDA receptor antagonist, has been reported to have both pro-seizure and anti-seizure effects in rat models of kindling [41].
Overall, most seizures associated with Alzheimer’s disease are responsive to AED monotherapy, often at low doses, which are preferred in an elderly population [2,30,42,43]. Whether low-dose levetiracetam (125mg twice a day), which seemed to improve hippocampal-based memory performance in patients with mild cognitive impair ment [23], is also effective at suppressing Alzheimer’s disease-associated seizures and epileptiform activity is unclear, but treatment with low-dose levetiracetam deserves consideration from a cognitive standpoint, particularly in patients with Alzheimer’s disease in whom seizures are mild or infrequent, and who have no history of status epilepticus. Levetiracetam should be used with caution in patients with advanced dementia and behavioural disturbances, because it can exacerbate agitation [44].
Treatment with many AEDs, particularly enzyme-inducing AEDs such as carbamazepine, phenytoin, and phenobarbital, is associated with decreased bone density. Therefore, long-term use of AEDs in elderly patients should be accompanied by routine examination of bone mineral density and supplementation with calcium, vitamin D, or other therapies.
References
- Sen A, Capelli V, Husain M (2018) Cognition and dementia in older patients with epilepsy. Brain 141(6): 1592-1608.
- Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL (2017) Epileptic activity in alzheimer's disease: causes and clinical relevance. Lancet Neurol 16(4): 311-322.
- Vezzani A, Granata T (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46(11): 1724-1743.
- Helmstaedter C, Alexander WJ (2017) Epilepsy and cognition-a bidirectional relationship? Seizure 49: 83-89.
- Gowers W (1881) Epilepsy and other chronic convulsive diseases: their causes, symptoms, & treatment. In: William Wood & Company, London, UK.
- Höller Y, Trinka E (2014) What do temporal lobe epilepsy and progressive mild cognitive impairment have in common? Front Syst Neurosci 8: 58.
- Duijn CMV, Chandra V, Fratiglioni L, Graves AB, Heyman A, et al. (1991) Medical history and the risk of Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 20 (Suppl 2): S36-42.
- Breteler MM, Groot R, Romunde LK, Hofman A (1995) Risk of dementia in patients with Parkinson’s disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol 142(12): 1300-1305.
- Hommet C, Mondon K, Camus V, Toffol B, Constans T (2008) Epilepsy and dementia in the elderly. Dement Geriatr Cogn Disord 25(4): 293-300.
- Imfeld P, Bodmer M, Schuerch M, Jick SS, Meier CR (2013) Seizures in patients with Alzheimer’s disease or vascular dementia: a population-based nested case-control analysis. Epilepsia 54(4): 700-707.
- Cook M, Baker N, Lanes S, Bullock R, Wentworth C, et al. (2015) Incidence of stroke and seizure in Alzheimer’s disease dementia. Age Ageing 44(4): 695-699.
- Cheng CH, Liu CJ, Ou SM, Yeh CM, Chen TJ, et al. (2015) Incidence and risk of seizures in Alzheimer’s disease: a nationwide population-based cohort study. Epilepsy Res 115: 63-66.
- Cretin B, Philippi N, Dibitonto L, Blanc F (2017) Epilepsy at the prodromal stages of neurodegenerative diseases. Geriatr Psychol Neuropsychiatr Vieil 15(1): 75-82.
- Amatniek JC, Hauser WA, Castaneda C, Jacobs DM, Marder K, et al. (2006) Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia 47(5): 867-872.
- Irizarry MC, Jin S, He F, Emond JA, Raman R, et al. (2012) Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch Neurol 69(3): 368-372.
- Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, et al. (2013) Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 70(9): 1158-1166.
- Zarea A, Charbonnier C, Lecrux AR, Nicolas G, Rousseau S, et al. (2016) Seizures in dominantly inherited Alzheimer disease. Neurology 87(9): 912-919.
- Volicer L, Smith S, Volicer BJ (1995) Effect of seizures on progression of dementia of the Alzheimer type. Dementia 6(5): 258-263.
- Subota A, Pham T, Jetté N, Sauro K, Lorenzetti D, et al. (2017) The association between dementia and epilepsy: a systematic review and meta-analysis. Epilepsia 58(6): 962-972.
- Huijbers W, Mormino EC, Schultz AP, Wigman S, Ward AM, et al. (2015) Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain 138(Pt 4): 1023-1035.
- Oh H, Jagust WJ (2013) Frontotemporal network connectivity during memory encoding is increased with aging and disrupted by beta-amyloid. J Neurosci 33(47): 18425-18437.
- Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M (2015) Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin 7: 688-698.
- Sperling RA, Laviolette PS, Keefe K, Brien JO, Rentz DM, et al. (2009) Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63(2): 178-188.
- Maestú F, Peña JM, Garcés P, González S, Bajo R, et al. (2015) A multicenter study of the early detection of synaptic dysfunction in mild cognitive impairment using magnetoencephalography-derived functional connectivity. Neuroimage Clin 9: 103-109.
- Braak H, Thal DR, Ghebremedhin E, Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70(11): 960-969.
- Jones TLS, Hyman BT (2014) The intersection of amyloid β and tau at synapses in Alzheimer’s disease. Neuron 82(4): 756-771.
- Ahmad S, Fowler LJ, Whitton PS (2005) Lamotrigine, carbamazepine and phenytoin differentially alter extracellular levels of 5-hydroxytryptamine, dopamine and amino acids. Epilepsy Res 63(2): 141-149.
- Yang XF, Rothman SM (2009) Levetiracetam has a time- and stimulation-dependent effect on synaptic transmission. Seizure 18(9): 615-619.
- Belcastro V, Costa C, Galletti F, Pisani F, Calabresi P (2007) Levetiracetam monotherapy in Alzheimer patients with late-onset seizures: a prospective observational study. Eur J Neurol 14(10): 1176-1178.
- Cumbo E, Ligori LD (2010) Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav 17(4): 461-466.
- Tekin S, Bingöl CA, Tanridağ T, Aktan S (1998) Antiglutamatergic therapy in Alzheimer’s disease-effects of lamotrigine. J Neural Transm (Vienna) 105(2-3): 295-303.
- Rowan AJ, Ramsay RE, Collins JF, Pryor F, Boardman KD, et al. (2005) New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology 64(11): 1868-1873.
- Tariot PN, Schneider LS, Cummings J, Thomas RG, Raman R, et al. (2011) Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry 68(8): 853-861.
- Fleisher AS, Truran D, Mai JT, Langbaum JBS, Aisenet PS, et al. (2011) Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology 77(13): 1263-1271.
- Tsiouris JA, Patti PJ, Tipu O, Raguthu S (2002) Adverse effects of phenytoin given for late-onset seizures in adults with Down syndrome. Neurology 59(5): 779-780.
- Rao SC, Dove G, Cascino GD, Petersen RC (2009) Recurrent seizures in patients with dementia: frequency, seizure types, and treatment outcome. Epilepsy Behav 14(1): 118-120.
- Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, et al. (2012) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149(3): 708-721.
- Gage SB, Moride Y, Ducruet T, Kurth T, Verdoux H, et al. (2014) Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 349: g5205.
- Shi JQ, Wang BR, Tian YY, Jun Xu, Li Gao, et al. (2013) Antiepileptics topiramate and levetiracetam alleviate behavioral deficits and reduce neuropathology in APPswe/PS1dE9 transgenic mice. CNS Neurosci Ther 19(11): 871-881.
- Hamberger MJ, Palmese CA, Scarmeas N, Weintraub D, Choi H, et al. (2007) A randomized, double-blind, placebo-controlled trial of donepezil to improve memory in epilepsy. Epilepsia 48(7): 1283-1291.
- Löscher W, Hönack D (1990) High doses of memantine (1-amino-3,5-dimethyladamantane) induce seizures in kindled but not in non-kindled rats. Naunyn Schmiedebergs Arch Pharmacol 341(5): 476-481.
- Cretin B, Sellal F, Philippi N, Bousiges O, Bitonto LD, et al. (2016) Epileptic prodromal Alzheimer’s disease, a retrospective study of 13 new cases: expanding the spectrum of Alzheimer’s disease to an epileptic variant? J Alzheimers Dis 52(3): 1125-1133.
- Sarkis RA, Dickerson BC, Cole AJ, Chemali ZN (2016) Clinical and neurophysiologic characteristics of unprovoked seizures in patients diagnosed with dementia. J Neuropsychiatry Clin Neurosci 28: 56-61.
- Ziyatdinova S, Gurevicius K, Kutchiashvili N, Bolkvadze T, Nissinen J, et al. (2011) Spontaneous epileptiform discharges in a mouse model of Alzheimer’s disease are suppressed by antiepileptic drugs that block sodium channels. Epilepsy Res 94(1-2): 75-85.
© 2021 Mirko Avesani. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






