- Submissions

Full Text
Gerontology & Geriatrics studies
Hypopituitarism Associated with a Microadenoma in an Elderly Patient
Pınar Tosun Tasar1*, Aykut Turhan2, Dogan Nasir Binici2, Sevnaz Sahin3 and Ozge Timur4
1Geriatrics Clinic, Erzurum Regional Training and Research Hospital, Erzurum
2Erzurum Regional Training and Research Hospital, Department of Internal Medicine,Erzurum
3Department of Internal Medicine ,Ege University School of Medicine, Izmir
4Ozge Timur,Erzurum Regional Training and Research Hospital, Erzurum
*Corresponding author: Pinar Tosun Tasar, Geriatrics Clinic, Erzurum Regional Training and Research Hospital, Erzurum
Submission: October 23, 2017; Published: December 06, 2017

ISSN : 2578-0093Volume1 Issue3
Abstract
Hypopituitarism is a rare condition in which the pituitary gland does not produce one or more of its hormones or does not produce them in sufficient quantities. Pituitary insufficiency in the elderly can present a genuine diagnostic and therapeutic challenge to the clinicians caring for these patients. Symptoms associated with partial or total hypopituitarism, such as fatigue, low muscle strength, and decreased libido, are not specific and can usually be attributed to the normal aging process. Destruction of pituitary cells accounts for more than 95% of cases of hypopituitarism. Pituitary micro adenomas are rare cause of hypopituitarism among elderly patients. Clinical diagnosis is easier with adenomas larger than 1cm in size because they exert pressure on the pituitary and cause symptoms. However, adenomas smaller than 1cm in size (microadenomas) usually do not cause symptoms and are therefore a very uncommon cause of hypopituitarism. In this case report, we present the rarely reported condition of hypopituitarism associated with microadenoma in an elderly patient.
Introduction
Hypopituitarism is a rare condition in which the pituitary gland does not produce one or more of its hormones or does not produce them in sufficient quantities. Pituitary insufficiency in the elderly can present a genuine diagnostic and therapeutic challenge to the clinicians caring for these patients. Symptoms associated with partial or total hypopituitarism, such as fatigue, low muscle strength, and decreased libido, are not specific and can usually be attributed to the normal aging process. The causes of hypopituitarism in the elderly are very similar to those in the younger population, and may be associated with pituitary or hypothalmic disease. The causes of hypopituitarism include pituitary macroadenoma (>1cm), pituitary microadenoma (<1cm), pituitary surgery, pituitary gland irradiation, peri- pituitary or hypothalmic tumor, autoimmune disease, lymphocytic hypophysitis, craniopharyngioma, infiltrative lesions, ischemic causes, meningioma, glioma, metastasis, granulomatous lesions, histiocytosis, hemochromatosis, subarachnoid hemorrhage, stroke, apoplexy, infectious causes, tuberculosis, HIV, traumatic brain injury, drugs, cytotoxic T lymphocyte antigen (anti-CTLA4) antibody treatment, opiates, rexinoids, and idiopathic causes [1].
Destruction of pituitary cells accounts for more than 95% of cases of hypopituitarism. Although pituitary microadenomas are an uncommon cause of hypopituitarism among elderly patients, larger adenomas exert pressure on surrounding anterior pituitary cells, resulting in insufficient production of one or more hormones [2]. Therefore, clinical diagnosis is easier with macroadenomas due to the symptoms caused by pressure effects on the pituitary. However, microadenomas usually do not cause pressure-related symptoms and are a very uncommon cause of hypopituitarism. This also makes it difficult to diagnose hypopituitarism associated with microadenoma. While pituitary adenomas in the elderly represent less than 10% of all pituitary adenomas, this rate is currently increasing due to improved health care and increased life expectancy [3,4].
In this case report, we present the rarely reported condition of hypopituitarism associated with microadenoma in an elderly patient.
Case Report
An 89-year-old female patient with no known chronic diseases experienced complaints of malaise and anorexia for one month. The patient presented to the geriatric outpatient clinic when her symptoms did not improve.
Upon questioning, she reported having no complaints other than anorexia, malaise, and fatigue. On physical examination, her overall condition was moderate-to-poor, body temperature was 36.8 °C, heart rate was 56/minute, respiratory rate was 18/ minute, and arterial blood pressure was 80/50mmHg. The patient exhibited dry skin and alopecia. Her medical and family histories were unremarkable. She was admitted to the geriatric inpatient unit with a prediagnosis of malnutrition.
The results of the first laboratory tests conducted after admission Table 1 suggested prerenal acute kidney injury (AKI) and intravenous fluid treatment was initiated.
Table 1: The patient’s blood analysis results at time of hospital admission.
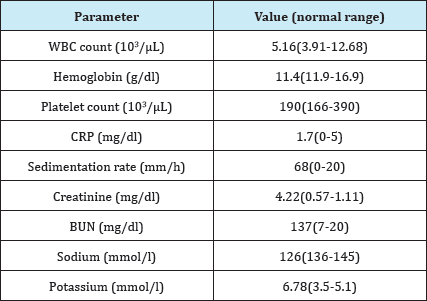
WBC- White blood cell; CRP- C-reactive protein; BUN- Blood urea nitrogen.
Table 2: The patient’s pituitary hormone levels.
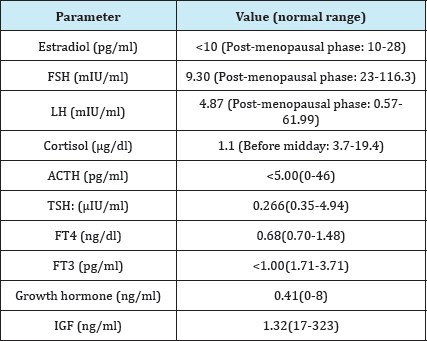
FSH- Follicle stimulating hormone; LH- Luteinizing hormone; ACTH- Adrenocorticotropic hormone; TSH- Thyroid stimulating hormone; FT4- Free thyroxine; FT3- Free triiodothyronine; IGF- Insulin-like growth factor.
Despite a downward trend in the patient s creatinine level, her hypotension did not improve. Electrocardiographic examination for differential diagnoses revealed no pathology. Due to the patient's persistent hypotension, pituitary hormone levels were assessed and the adrenocorticotropic hormone (ACTH) stimulation test was performed (Table 2). Based on the results of these tests, the patient was diagnosed with hypopituitarism. Treatment with methylprednisolone (60mg, 1mg/kg) was initiated. The hypotension resolved on the second day of treatment and oral nutrition was resumed. On the fourth day of treatment, levothyroxine sodium was initiated and methylprednisolone was gradually reduced.
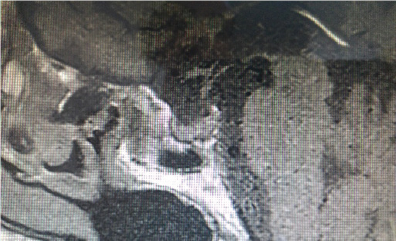
Magnetic resonance imaging (MRI) of the pituitary gland for differential diagnosis of hypopituitarism revealed a microadenoma 8x3 mm in size in the posterior aspect of the anterior pituitary. The patient was diagnosed with hypopituitarism associated with micro adenoma (Figures 1&2). No pathology was detected in visual field testing. On day 14 of treatment with methylprednisolone and levothyroxine sodium, the patient's bradycardia, hypotension, and hyponatremia had fully resolved, and her general condition and laboratory values had were improved. She was discharged and followed on an outpatient basis.
Figure 1: The patient was diagnosed with hypopituitarism associated with microadenoma.
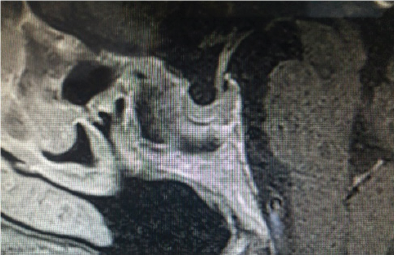
Figure 2: The patient was diagnosed with hypopituitarism associated with microadenoma.
Discussion
Hypopituitarism may develop rapidly or slowly depending on the underlying etiology and may involve one, multiple, or all of the pituitary hormones. The latter is called panhypopituitarism. Clinical signs and symptoms may be secondary to hormonal insufficiency or pressure symptoms caused by pituitary or hypothalamic tumor.
Common pressure symptoms are changes in vision, typically bitemporal hemianopsia, and headache. In addition, hyperprolactinemia is a common finding with macroadenomas that do not produce prolactin, due to pituitary stalk compression and impaired dopaminergic inhibition [5]. High prolactin, through either direct or indirect inhibition of GnRH, may induce secretion of luteinizing hormone, which leads to low levels of testosterone and subsequent reduced libido, thus causing male sexual dysfunction.
Hypopituitarism generally causes nonspecific symptoms which are also signs of normal aging, such as fatigue, low energy, weight loss, and reduced appetite. Therefore, hypopituitarism is not easily diagnosed in the older population. Details of the clinical presentation of specific hormonal deficiencies are discussed below [6].
Nearly all the symptoms of ACTH deficiency are due to cortisol deficiency. This is the most critical of all pituitary hormone deficiencies. It can manifest with orthostatic hypotension, fatigue, nausea, vomiting, dizziness, hypoglycemia, or nonspecific abdominal pain.
Secondary hypothyroidism is rare. Clinical findings like low energy levels, weight gain, cold intolerance, constipation, dry skin, alopecia, and bradycardia occur due to low thyroxine (T4) level.
Diagnosis of male hypogonadism is based on a combination of low androgen levels and symptoms of hypogonadism. The testicles are soft and have reduced volume. Other signs include slow facial hair growth, erectile dysfunction, decreased libido, decreased muscle and bone mass, or anemia, and erythropoiesis may be reduced [7]. Older women with hypogonadism exhibit osteoporosis, decreased libido, vaginal dryness, and chest atrophy, which are indistinguishable from menopausal symptoms [1].
The activity of the GH-IGF-1 axis decreases in parallel with the normal age-related reduction in insulin-like growth factor 1 (IGF-1) levels seen in older patients [8]. Some authors have attributed this reduction to decreases in GHRH-secreting neurons or somatostatinergic hyperactivity [9]. Clinically, patients may experience changes in body composition such as increased fat mass, reduced muscle mass and strength, fatigue, or lower bone mineral density.
Antidiuretic hormone (ADH) deficiency manifests as polyuria and polydipsia with a continuous desire to drink cold beverages, and is associated with diabetes insipidus [1].
A diagnosis of hypopituitarism is confirmed biochemically by baseline hormone levels or by dynamic function tests [9]. However, there are limited data regarding the validity of these diagnostic tests in the older population. Most guidelines are based on the findings observed in younger patients.
The management of hypopituitarism is based on treating the underlying pathology when possible, and hormone replacement therapy when needed. The use of therapeutic methods such as drugs, surgery, or radiotherapy, either singly or in combination, may be required in order to achieve an optimal result in the treatment of a pituitary tumor [10-12]. For some tumors, surgery may be necessary to reduce pressure effects on structures the optical chiasma or cavernous sinus. On the other hand, immediate neurosurgery may be needed to prevent compression of nearby structures (e.g.: the optic chiasma and nerves), as in pituitary apoplexy [13,14]. Finally, it is crucial to identify cases of life- threatening hypopituitarism and promptly initiate treatment with appropriate hormonal replacement therapies. The goal of hormone replacement therapy is to maintain hormonal physiology to the extent possible and prevent symptoms associated with deficiency. In patients with panhypopituitarism or isolated ACTH deficiency, glucocorticoid replacement is of vital important. Hydrocortisone is the preferred hormone; other alternatives include prednisone, methylprednisolone, cortisone, and dexamethasone, which basically differ in terms of duration of action and glucocorticoid vs mineralocorticoid effects.
Thyroxine replacement is the treatment for secondary hypothyroidism. It is very important to start at low doses (0.25- 0.5|ig/kg) with elderly patients [15,16]. Levothyroxine can be gradually increased every 3-4 weeks after the initial dose is tolerated. Factors such as reduced metabolic clearance, decreased body mass and other comorbid conditions are among the reasons for starting treatment with low thyroxine doses in the elderly [17]. Most importantly, thyroxine replacement must not be initiated before adrenal insufficiency is adequately treated. In patients with both hypocortisolism and hypothyroidism, treating only the hypothyroidism may increase the clearance of cortisol, which is already scarce, which exacerbates the cortisol deficiency and may in some cases trigger an adrenal crisis [18,19].
The risk of cardiovascular disease and osteoporosis increases in normal postmenopausal women. It was initially believed that hormone replacement therapy could lower these risks. However, various trials conducted in the last 4-5 years were unable to demonstrate this beneficial effect, and the benefit/risk ratio has been debated. The procoagulant effect is believed to be cardioprotective, especially in older women after menopause. However, the long-term effects of estrogen replacement on cardiovascular morbidity or the development of breast cancer in older women with hypopituitarism are unknown. Androgen replacement is the standard therapy for male hypogonadism. Testosterone therapy is recommended for symptomatic males in order to improve sexual function and bone mineral density. Androgen therapy has also been shown to positively impact anthropometric measurements and lipids [20,21]. Recombinant growth hormone (rhGH) has been available since 2005. Growth hormone deficiency in adults may result in reduced IGF-1, increased fat mass, abnormal bone turnover, and an unfavorable lipid profile compared to healthy adults, and its replacement is believed to reverse these effects [22,23].
Patients with diagnosed ADH deficiency may be treated with adequate oral intake of fluids in mild cases, while desmopressin may be required in severe cases. Desmopressin may be administered via oral, nasal, or parenteral routes [24].
Conclusion
Hypopituitarism in the elderly can mimic the symptoms of normal aging. Therefore, clinicians must be vigilant and diagnose the disease before symptoms progress. Changes in hormonal axes can be assessed by morning cortisol, thyroid stimulating hormone, free T4, IGF-1, luteinizing hormone, follicle stimulating hormone, sex hormones and, if necessary, ACTH stimulation test. When necessary, glucocorticoids and initially low-dose thyroid hormone replacement should be initiated. The benefits and risks of sex hormone replacement should be addressed for each patient individually. Growth hormone replacement is controversial and must be closely monitored. Regular follow-up of these patients is necessary to evaluate the treatment and definitively assess the associated risks. Long-term studies that assess the benefits and risks of pituitary hormone replacement, particularly of sex and growth hormones, are needed in order to better guide physicians' treatment decisions and achieve optimal outcomes for patients.
References
- Antonopoulou M, Sharma R, Farag A, Banerji MA, Karam JG, et al. (2012) Hypopituitarism in the elderly. Maturitas 72(4): 277-285.
- Lamberts SW, De Herder WW, Van Der Lely AJ (1998) Pituitary insufficiency. The Lancet 352(9122): 127-134.
- Cohen DL, Bevan JS, Adams CB (1989) The presentation and management of pituitary tumours in the elderly. Age Ageing 18(4): 247-252.
- Turner HE, Adams CB, Wass JA (1999) Pituitary tumours in the elderly: a 20 year experience. Eur J Endocrinol 140(5): 383-389.
- Ascoli P, Cavagnini F (2006) Hypopituitarism. Pituitary 9(4): 335-342.
- Toogood AA, Stewart PM (2008) Hypopituitarism: clinical features, diagnosis, and management. Endocrinol Metab Clin North Am 37(1): 235-261.
- Daniell HW (2006) Erythropoietin resistance during androgen deficiency. Arch Intern Med 166(17): 1923-1924.
- Florini JR, Prinz PN, Vitiello MV, Hintz RL (1985) Somatomedin-C levels in healthy young and old men: relationship to peak and 24-hour integrated levels of growth hormone. J Gerontol 40(1): 2-7.
- Ghigo E, Arvat E, Gianotti L, Ramunni J, Divito L, et al. (1996) Human aging and the GH-IGF-I axis. J Pediatr Endocrinol Metab 9 (Suppl 3): 271278.
- Colao A, Annunziato L, Lombardi G (1998) Treatment of prolactinomas. Ann Med 30(5): 452-459.
- Lissett CA, Peacey SR, Laing I, Tetlow L, Davis JR, et al. (1998) The outcome of surgery for acromegaly: the need for a specialist pituitary surgeon for all types of growth hormone (GH) secreting adenoma. Clin Endocrinol (Oxf) 49(5): 653-657.
- Gittoes NJ, Bates AS, Tse W, Bullivant B, Sheppard MC, et al. (1998) Radiotherapy for non-function pituitary tumours. Clin Endocrinol (Oxf) 48(3): 331-337.
- Hasegawa Y, Yano S, Sakurama T, Ohmori Y, Kawano T, et al. (2011) Endoscopic surgical treatment for pituitary apoplexy in three elderly patients over the age of 80. Acta Neurochir Suppl 111: 429-433.
- Turgut M, Ozsunar Y, Bajak S, Guney E, Kir E, et al. (2010) Pituitary apoplexy: an overview of 186 cases published during the last century. Acta Neurochir (Wien) 152(5): 749-761.
- Roos A, Linn Rasker SP, van Domburg RT, Tijssen JP, Berghout A, et al. (2005) The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med 165(15): 1714-1720.
- Davis FB, LaMantia RS, Spaulding SW, Wehmann RE, Davis PJ, et al. (1984) Estimation of a physiologic replacement dose of levothyroxine in elderly patients with hypothyroidism. Arch Intern Med 144(9): 17521754.
- Sawin CT, Herman T, Molitch ME, London MH, Kramer SM, et al. (1983) Aging and the thyroid. Decreased requirement for thyroid hormone in older hypothyroid patients. Am J Med 75(2): 206-209.
- Wang G, Cai C, Wu B (2010) Thyroid hormones precipitate subclinical hypopituitarism resulted in adrenal crisis. J Am Geriatr Soc 58(12): 2441-2442.
- Graves L, Klein RM, Walling AD (2003) Addisonian crisis precipitated by thyroxine therapy: a complication of type 2 autoimmune polyglandular syndrome. South Med J 96(8): 824-827.
- Zgliczynski S, Ossowski M, Slowinska Srzednicka J, Brzezinska A, Zgliczynski W, et al. (1996) Effect of testosterone replacement therapy on lipids and lipoproteins in hypogonadal and elderly men. Atherosclerosis 121(1): 35-43.
- Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc 58(6): 1134-1143.
- Monson JP, Abs R, Bengtsson BA, Bennmarker H, Feldt Rasmussen U, et al. (2000) Growth hormone deficiency and replacement in elderly hypopituitary adults. KIMS Study Group and the KIMS International Board. Pharmacia and Upjohn International Metabolic Database. Clin Endocrinol Oxf 53(3): 281-289.
- Abrams P, Boquete H, Fideleff H, Feldt-Rasmussen U, Jonsson PJ, et al. (2008) GH replacement in hypopituitarism improves lipid profile and quality of life independently of changes in obesity variables. Eur J Endocrinol 159(6): 825-832.
- Chanson P, Salenave S (2011) Treatment of neurogenic diabetes insipidus. Ann Endocrinol (Paris) 72(6): 496-499.
© 2017 Pınar Tosun Tasar, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






