- Submissions

Full Text
Gerontology & Geriatrics studies
The Aging Brain: Recent Research and Concepts
Vinod Nikhra*
Hindu Rao Hospital, India
*Corresponding author: Vinod Nikhra, Senior Chief Medical Officer and Teaching faculty, Hindu Rao Hospital, A public sector thousand-bed multi specialty Hospital, India
Submission: October 23, 2017; Published: December 05, 2017

ISSN : 2578-0093Volume1 Issue3
Abstract
Overview-neuro cognitive aging: Among all body organs, the aging of human brain is most incapacitating with its fallouts on quality of life, general health and psychosocial implications. There are progressive aging changes in the brain with increasing chronological age. However, at the individual level rate and types of changes are variable. The aging of brain entails several structural, bio-chemical and functional changes in the brain as well as various cognitive changes. The changes that may affect cognition and behavior occur at the molecular, intracellular, intercellular and neuronal tissue levels. Further, as suggested by the research in animal models, with aging there are distinct changes in the expression of genes at the neuronal level. In fact, aging is a major risk factor for the common neurodegenerative diseases, which include mild cognitive impairment (MCI), Alzheimer's disease (AD) and Parkinson's disease (PD).
Morphological alterations: As a person gets older, changes occur in the brain. Certain parts of the brain shrink, especially those related to memory and learning, and other complex cognitive activities. The injury due to reactive oxygen species (ROS) at micro-level and ensuing inflammation and degeneration compromise the neuronal ability to function, affecting the three important processes: communication, metabolism and repair/ regeneration. The other types of brain cells, called glial cells, which play various critical roles apart from supporting neurons also suffer changes due to aging process. Blood circulation in the brain decreases due to multiplicity of factors, including changes in cerebral vasculature, and is a likely cause of cognitive decline. The human brain consumes about 20 percent of the body's oxygen, and the micro- and mini-vascular disturbances (mini-strokes) are common with aging arteries, and cause a cumulative and progressive damage.
The cognitive impairment: The cognitive abilities change throughout life, first as a result of brain maturation and later with aging of brain cells and their multitudes of complex interconnections. As people age, their movements and reflexes slow and the hearing and vision weaken. An important issue is how normal brain aging transitions to pathological aging, giving rise to neurodegenerative disorders. The toxic protein aggregates have been identified as potential contributory factors, including amyloid beta-protein in AD, tau in front temporal dementia, and Lewy bodies in PD. But, despite dementia and other neurodegenerative disorders associated with aging, the advanced imaging techniques have revealed that even into late seventies, the brain is able to regenerate and produce new neurons, and restructure complex neuronal circuits. Thus, a chance of regeneration and repair exists even in the aging brain.
Measures to retard the cognitive aging: The level of education and lifetime of intellectual effort, which improve cognitive skills, seem to protect brain against aging as well. The brain cells can grow and regenerate and the learning can improve throughout life. Though the aging is not genetically programmed, genes influence the aging of brain in multiple ways. The CETP gene is important in this regard and its I405V variant may influence general cognitive function and pathogenesis of AD. The gene expression, in turn, is influenced by factors like a healthy lifestyle, exercise, dietary and alcohol intake, and mental activity. The calorie restriction (CR) and CRAN appear to have a positive impact on cognitive function and aging. A better understanding of the aging of brain can be viewed as a key to an improved quality of life in a world where people will live longer in near future.
Keywords: Aging brain; Cognitive reserve; Neuronal aging; Neurotransmitters; Essential cognitive decline; Neurodegenerative disorders; Mild cognitive impairment; Alzheimer's disease; Front temporal dementia; Parkinson's disease; Cognitive protection; Reactive oxygen species; Calorie restriction; CRAN
Introduction
The aging of the brain is due to pathophysiological changes over the period of individual lifespan. Further, the aging in general, is not genetically programmed. In fact, it can be compared with wear and tear plus the efforts at biological level for repair and adaptation. Various insults, infective and inflammatory posed to the human- being lead to subcellular, cellular and tissue abnormalities resulting in neuronal adaptation, senescence and degeneration culminating into the complex phenomenon of aging of brain (Figure 1). The latter manifests differently in different individuals depending on their genomic constitution, childhood, early and late adult life events and exposure to the total biological environment.
Essential cognitive decline
With aging, the cognitive abilities decline. Though, the decline may not be apparent in form of impairment of activities of daily living (ADL), as changes are gradual and subtle. There occurs cognitive slowing, which may be evident on attention tasks or when it is necessary to multitask or when switching from one task to another. Driving is such a task and cognitive slowing is a contributing factor in elderly people's higher rate of automobile accidents [1].
Figure 1: The Concepts of Aging of Brain.

The ability to keep multiple pieces of information in mind at the same time is another skill that declines with aging. The memory also declines, but the exact nature of the decline depends on the particular type of memory. To recall a new event or information, the brain must register the information, store it, and then retrieve it when needed. The ability to recall new information gradually declines after 40s. The older adults are less likely than young adults to freely recall most of the new information, though they can recognize when the content is mentioned.
The language skills develop during young years and are retained during adulthood. But, recalling a familiar name or a particular word may become tardy for older adults. The visual perceptual and scanning abilities, and spatial relationships show decline with age. Executive functioning, such as conceptualizing a problem, making appropriate decisions, and planning and carrying out effective actions slowdown in older adults [2]. The older adults are more likely to be fooled by deceptive advertising and at greater risk of falling prey to fraud. Language and vocabulary are, though, well retained throughout the lifespan. In fact, vocabulary continues to improve into middle age. The age-resistant cognitive skills are strengthened by experience, including situations that require reasoning and judgment. The older adults often have a better overview of a situation or single event, than younger people, because of their greater experience and emotional tranquility [3].
But, the cognitive operations do not exist in isolation. Multiple cognitive skills, such as attention, memory and reasoning, are involved in performing ADL. The social behavior relies on a combination of cognitive and emotional factors, and the influence of aging on these factors is multifaceted. The brain aging interferes with performance when information is acquired in an unfamiliar situation and needs to be processed quickly. In familiar situations, older adults as compared to younger adults, tend to make more accurate interpretations of the behaviors of others when prior experience and knowledge helps to focus attention.
Physiological Vs abnormal brain aging
Each neuron has a cell body and a number of processes called dendrites that extend in many directions to join other neurons for receiving signals. Each neuron has an axon that transmits signals;these axons make up the white matter in the brain. A number of other changes occur in the brain with aging. The damage to white matter tracts with aging contributes to decreased brain size and has been associated with slower information-processing and difficulty in recalling information. With aging, the size and complexity, and the efficiency of communication between neurons become less effective and leads to various symptoms and signs of cognitive dysfunction (Figure 2).
Figure 2: The Physiological Aging and Cognitive Decline.
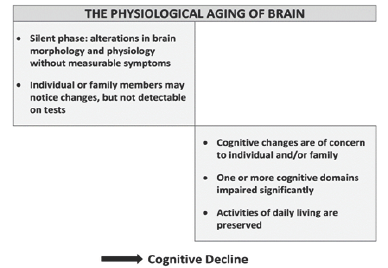
There are individual differences in aging related cognitive decline. For some people, cognitive decline with aging is mild. It is explainable by a factor is called the cognitive reserve [4]. Those more intelligent at a young age, or having better cognitive maintenance through education, occupation, or stimulating activities, having right genes and social network and friends, and enjoying activities with others, having a purpose in life retain cognitive skills with aging better than those who are less accomplished, they have been shown to be associated cognitive reserve. The level of education and lifetime of intellectual effort, which improve cognitive skills, seem to protect brain against aging as well. The intellectual stimulus leads the brain cells to grow and regenerate, replenish loss of neurotransmitters and retards the neuronal aging, and improves the cognitive reserve.
With aging, the changes in the pattern of stimulation of neural networks, cause increased activation in some areas and decreased activation in others. Studies reveal that when an elderly person performs a cognitive task at the same level as that of a young adult, more brain activity is needed to maintain cognitive performance. The physical frailty with age is accompanied with proportional cognitive decline, and can be leveled as normal aging process [5].
Both, the physiological brain aging and neurodegenerative disorders involve impaired energy metabolism and oxidative damage. The neuronal plasma membrane contains redox enzymes that provide electrons for energy metabolism and recycling of antioxidants such as coenzyme Q and a-tocopherol. The pathology of aging and age-related diseases involves oxidative stress at an early stage. The reactive oxygen species (ROS) produced during mitochondrial oxidative phosphorylation are associated with damage to DNA, lipids, and proteins [6]. The accumulation of mitochondrial DNA mutations, induces impairment of mitochondrial function, leading to a shortage of ATP supply and energy metabolism.
The research indicates that mitochondria have a central role in aging-related neurodegenerative diseases. Mutations in mitochondrial DNA and oxidative stress both contribute to neuronal aging and neuro degeneration [7]. The mitochondrial dysfunction and oxidative damage are major contributors to neuronal loss. Free radicals, typically generated from mitochondrial respiration, cause oxidative damage of nucleic acids, lipids, carbohydrates and proteins. Though, the overall mechanism by which oxidative damage causes neuronal death is not well understood [8].
The neurodegenerative disease is an umbrella term for a range of conditions which primarily affect the neurons in the human brain and involve progressive loss of structure and function, leading to progressive degeneration and death of neurons, and include AD, PD, amyotrophic lateral sclerosis and Huntington's disease [9]. The neurodegenerative process trigger neuronal cell death. The programmed cell death (PCD) plays a critical role and is influenced by both anti-PCD and pro-PCD modulators. There are least three different forms of PCD: Type I-nuclear or apoptotic, Type II- autophagic, and Type III-cytoplasmic. The PCD pathways may also be activated by various insults, such as misfolded proteins, reactive oxygen and nitrogen species, mitochondrial-complex inhibition, calcium entry, excitotoxicity, trophic-factor withdrawal, and death- receptor activation.
Neuronal loss is a relatively late event in the neurodegenerative process, and the neuronal death is preceded by early functional alterations like electrophysiological deficits and cellular-stresspathway activation, micro an atomical deficits such as neurite retraction and synapse loss, and somal atrophy [10]. The leading neurodegenerative disease, AD is characterized by loss of neurons and synapses in the cerebral cortex and certain subcortical areas. This loss results in gross atrophy of the affected regions, including degeneration in the temporal lobe and parietal lobe, and parts of the frontal cortex and cingulate gyrus [11].
The apoptosis is a form of PCD through extrinsic apoptotic pathways, when factors outside the cell activate cell surface death receptors, and intrinsic apoptotic pathways involving mitochondrial release of cytochrome c or endoplasmic reticulum malfunctions. Each of these finally lead to the activation of caspases (cysteine- aspartic acid proteases), which are of two types of caspases: initiators and effectors. The initiator caspases cleave the inactive forms of effector caspases. The activated effectors, in turn, cleave cell proteins resulting in apoptotic initiation. These biochemical events lead to a characteristic damaged cell morphology and death [12].
The astrocytes are fundamental for homoeostasis, defense and regeneration. There are dynamic astrocyte-neuron interactions involving calcium signaling and sodium signaling following release of neurotransmitters [13]. There occurs loss of astroglial function and astroglial reactivity with age and contributes to the aging of the brain as well as neurodegenerative diseases. The physiological changes in astroglia with aging and neuro degeneration are heterogeneous. The astrocytes undergo degeneration and atrophy at the early stages of pathological progression as documented in AD animal models, which possibly alter the homeostatic reserve of the brain and contribute to early cognitive deficits [14].
The line of demarcation between physiological and pathological cognitive changes is not clear. It is difficult to outline how much cognitive decline is purely the result of aging of an otherwise healthy brain. A disproportionate cognitive decline may not be because of physiological cognitive aging and the older adults in cognitive studies are likely to include people with undetected Alzheimer's disease (AD), cerebral vascular dementia, and other neurodegenerative diseases. With physiological brain aging, there may occur build-up of a small neuronal protein fragment called amyloid beta. The studies suggest that amyloid plaques trigger the build-up of abnormal tau, which causes neuronal loss within brain cells in AD patients.
Morphological Alterations in Aging Brain
Cellular and sub-cellular alterations
There occur distinct morphological changes in brain with aging. These are progressive changes in the aging brain, though at individual level the rate and extent of changes is variable. Simultaneously, there are occurring changes in vasculature and white matter. In general, the aging is associated with several structural, biochemical, and functional alterations in the brain. These alterations may be associated several neuro cognitive changes differing in severity. In fact, there is considerable variability in the age at which cognitive abilities peak and decline throughout life [15].
The changes that may affect cognition and behavior occur at the molecular, intracellular, intercellular and neuronal tissue levels. Further, as suggested by the aging research animal models, there are distinct changes in the expression of genes at the neuronal level. In fact, aging is a major risk factor for the common neurodegenerative disorders, which include MCI, AD and PD.
Cerebral volume loss and ventriculomegaly
The various tissues in the brain differ in susceptibility to age- induced changes [16]. As documented by the CT and MRI studies, with aging the cerebral ventricles expand (ventriculomegaly) and there occur regional decreases in cerebral volume [17]. Further, the regional volume reduction is not uniform; some brain regions shrink at a rate of up to 1% per year, whereas others remain relatively stable until late in the life. Thus, some areas such as the cingulate gyrus, and occipital cortex surrounding the calcarine sulcus are spared from the decrease in grey matter density. The proposed mechanisms of differential brain aging include neurotransmitter systems, stress and hormonal changes, micro vascular changes, calcium homeostasis, and demyelination [18]. In general, the volume of the brain declines with age at a rate of about 5% per decade after age 40 and rate of decline increases with age over 70 [19].
With the loss of cerebral volume there occurs thinning of the cortex. The MRI studies have noted a decrease in grey matter volume between adulthood and old age, whereas white matter volume increases from age 19-40, and declines thereafter [20]. The areas such as the insula and superior parietal gyri are especially vulnerable to age-related grey matter loss in older adults. Most of the decrease in grey matter density occurs in dorsal, frontal and parietal lobes on both interhemispheric and lateral brain surfaces.
Aging related loss of grey matter density in the temporal cortex appears more predominantly in the left versus right hemisphere, involving cortical language areas. These anterior language cortices have been found to mature and decline earlier than the more posterior language cortices. The width of related sulcus also increases with age [21]. Further, there are sex differences in the loss of gyri and increase in sulci [22].
The white matter loss
The white matter declines with age and the myelin sheath deteriorates after around the age of 40 even in normal brain aging. The late myelinating regions of the frontal lobes are most affected by white matter lesions [23]. It has been suggested that leukoariosis or white matter lesions increase with age and may indicate subclinical ischemia. A study looking at cortical volume and white matter volume in subjects aged 50 to 81 years, documented an association between reduction in prefrontal cortical volume, increased sub- cortical white matter lesions, and a decreased executive function with aging [24].
Age-related changes in neurons
The changes do not occur to the same extent in all brain regions. The temporal lobe, cerebellar vermis, cerebellar hemispheres, and hippocampus reduce in volume. The prefrontal cortex is most affected and the occipital least. Frontal and temporal lobes are most affected in men compared with the hippocampus and parietal lobes in women. The shrinking of the grey matter is frequently reported to stem from neuro degeneration or neuronal cell death [25].
There occur various region-specific changes in the morphology of neurons. The dendritic arbors and dendritic spines of cortical pyramidal neurons decrease in size and/or number in specific regions and layers of cortex as a result of aging [26]. There has been shown about 46% decrease in spine number and spine density in humans older than 50 compared with younger individuals. In animal research, the older monkeys show 50% loss in spines on the apical dendritic tufts of pyramidal cells in prefrontal cortex, compared with the young ones [27].
Compared to cerebral areas, the cerebellum is protected from aging effects. Thus, the cerebellum exhibits fewer neuropathological manifestations of age related deficits compared to other brain regions.
Loss of neural circuits and brain plasticity
The plasticity refers to the brain's ability to change structure and reassign functions [28]. With aging there occur changes leading to plasticity deficits [29]. The age-related plasticity deficit has been related to age-induced alterations in calcium regulation [30]. It appears that the alterations in calcium regulation influence the neuronal ability to generate and propagate action potentials, which in turn affects the ability of the brain to modify its structure and reassign the functions [31]. Further, neurotransmitters like brain-derived neurotrophic factor (BDNF) and serotonin (5-hydroxytryptamine, 5-HT) appear to play a role in neuroplasticity [32].
The neural circuits of some areas of brain are more vulnerable to aging than others. Especially vulnerable neural circuits are the hippocampal and neocortical circuits. The age-related cognitive decline may in part be due to alterations at synaptic levels. The cognitive deficitalso appears to be due to physiological and biochemical factors such as changes in enzymatic activity, chemical messengers, or gene expression in cortical circuits [33].
Another factor to consider with regard to the aging brain, its plasticity and cognitive performance is the hormonal influence. The changes in sex hormones occur with aging, in women at menopause in and in men at andropause. Women also have higher prevalence of failing memory and a higher incidence of AD [34]. It has been noted that it may improve with estrogen therapy which increases dopaminergic responsivity [35] and may be protective in AD [36]. Growth hormone levels also decline with age and its supplementation appears to be associated with improvement in cognitive function [37].
Neurofibrillary tangles
An important difference between normal brain aging and pathological changes, is the presence and distribution of neurofibrillary tangles [38]. The neurofibrillary tangles are composed of paired helical filaments. In normal, non-pathological aging, the number of tangles in each affected tissues is relatively low and restricted to the olfactory nucleus, para-hippocampal gyrus, amygdala and entorhinal cortex. With normal aging, there is a small butgeneral increase in the density of tangles. Whereas, in the pathological brain aging, representing the neurodegenerative entity, neurofibrillary tangles are commonly found with amyloid plaques in AD patients.
Etiogenesis of Changes of the Aging Brain
Role of oxidative stress
The cognitive decline has been attributed to oxidative stress, cytokines, inflammatory reactions and neuronal degeneration, and changes in the cerebral microvasculature [39]. The Oxidative stress is the cumulative damage done by reactive oxygen species (ROS) or free radicals released fromvarious metabolic oxidation processes and inadequately neutralized by physiological antioxidant system (Figure 3). Compared to other tissues in the body, the brain is more sensitive to oxidative damage and the increased oxidative stress has been linked with cognitive impairment. The difference in oxidative damage related to various lifestyle factors has been associated with individual differences in cognition impairment in healthy elderly people from mild cognitive impairment to severe decline and predisposition to neurodegenerative disorders [40].
Figure 3: Theories and Concepts of Neuro degeneration.

The main contributors for oxidative stress are protein and lipid oxidation, and oxidative reactions in nuclear and mitochondrial DNA. Oxidative stress can damage DNA replication and inhibit repair through various complex processes, including telomere shortening. As the telomere length is partly inheritable, it can be responsible for individual differences in the age of onset and extent of cognitive decline [41].
Genetic changes
The variation in the effects of aging among individuals can be attributed to both genetic and epigenetic factors. With aging, the brain shows a decline in cognitive function and alterations in gene expression. This modulation in gene expression may be due to oxidative DNA damage at promoter regions in the genome. Further, this has been endorsed by the research in animal models, which documents distinct changes in the expression of genes at the neuronal level with aging [42].
The DNA damage increasingly accumulates with age in the brain and includes the oxidized nucleoside 8-hydroxy-deoxy-guanosine (8-OHdG), single- and double-strand breaks, DNA-protein cross links and malondialdehyde (MDA) adducts [43]. MDA is a highly reactive compound and a marker for oxidative stress and a measure of free radical activity. It has been reported in animal studies that the young 4-day-old rats have about 3,000 single-strand breaks and 156 double-strand breaks per neuron, whereas in rats older than 2 years the level of damage is increased to about 7,400 singlestrand breaks and 600 double-strand breaks per neuron [44]. The mitochondrial changes with aging also affect neurons [45].
There are certain genes that are down-regulated over the age of 40 and include GluR1 AMPA receptor subunit, NMDA R2A receptor subunit (involved in learning), subunits of the GABA-A receptor, calmodulin 1 and CAM kinase II alpha (genes involved in longterm potentiation), and calcium signaling genes, synaptic plasticity genes, synaptic vesicle release and recycling genes. Whereas, certain genes which are upregulated include the genes associated with stress response and DNA repair. The transcriptional profiles of the human frontal cortex of individuals ranging from 26 to 106 years of age have been studied. Lu identified of a set of genes whose expression was altered after age 40, and that the promoter sequences of these particular genes accumulated oxidative DNA damage, including 8-OHdG, with age. They concluded that DNA damage may reduce the expression of selectively vulnerable genes involved in learning, memory and neuronal survival, initiating a pattern of brain aging that starts early in life [46].
Neurosignaling and neurotransmitters
Counter-regulatory mechanisms: Neurotransmitters tend to regulate each other's action and release. A mild imbalance in the mutual regulation has been linked to temperament variations in healthy people. Whereas, severe imbalances or disruptions in neurotransmitter systems have been associated with disorders like depression, insomnia, attention deficit hyperactivity disorder (ADHD), anxiety, memory loss, Parkinson’s disease etc. The chronic stress can be a contributor to neurotransmitter system changes. The genetics also plays a role in neurotransmitter activities.
A neuron transports information by a nerve impulse called the action potential. When an action potential arrives at the synapse's presynaptic terminal button, it may stimulate release of neurotransmitters. These neurotransmitters are released into the synaptic cleft to bind onto the receptors of the postsynaptic membrane and influence another neuron, either in an inhibitory or excitatory way, with the probability that it will induce an action potential. Each neuron receives a multitude of excitatory and inhibitory signals every second. The type I (excitatory) synapses are typically located on the shafts or the spines of dendrites, whereas type II (inhibitory) synapses are typically located on a cell body.
Figure 4: The Brain Neurotransmitters..

Brain neurotransmitter systems: The aging process entails various biochemical changes in brain. The recent research has identified a number of neurotransmitters, as well as their receptors which exhibit alterations in various regions of the brain with the aging process.The major neurotransmitter systems include the noradrenaline (norepinephrine) system, the dopamine system, the serotonin system, and the cholinergic system, among others (Figure 4). The trace amines have a very significant effect on neurotransmission in monoamine pathways (i.e., dopamine, histamine, norepinephrine, and serotonin pathways) throughout the brain. The most prevalent transmitter in human brain is glutamate, which is excitatory at over 90% of the synapses. The next most prevalent neurotransmitter is Gamma-Aminobutyric Acid, or GABA, which is inhibitory at more than 90% of the synapses that do not use glutamate. In addition, there are over 50 neuroactive peptides e.g. p-endorphin. Certain other transmitters are used in fewer synapses, albeit they are important.
A. Dopamine and Serotonin: The neurotransmitters most important with regard to aging are dopamine and serotonin. They appear to be responsible for the regulation of synaptic plasticity and neurogenesis in the adult brain [47]. The serotonin and BDNF levels also fall with aging. Another factor, monoamine oxidase, increases with age and may be responsible for excess liberation of free radicals that exceed the inherent antioxidant reserves [48].
B. Dopamine: Dopaminergic pathways regulate cognitive processes and behaviors such as arousal (wakefulness),aversion, cognitive control and working memory (co-regulated by norepinephrine), emotion and mood, motivation, motor function, positive reinforcement, reward (primary mediator), sexual arousal, orgasm, and refractory period (via neuroendocrine regulation). The dopamine functions in the brain include regulation of motor behavior, pleasures related to motivation and emotional arousal. It also plays a critical role in the reward system. Parkinson's disease has been linked to low levels of dopamine and schizophrenia has been linked to high levels of dopamine.
There occur age-related changes in brain in dopamine synthesis, binding and receptors [49]. In normal human volunteers, positron emission tomography (PET) studies show a significant age-related decline in dopamine synthesis in the striatum and parastriatal regions [50]. Also, there is a significant age-related decrease in D1, D2 and D3 dopamine receptors [51].
Dopamine levels decline by about 10% per decade from early adulthood and have been associated with declines in cognitive and motor performance [52]. Possibly, the dopaminergic pathways between the frontal cortex and the striatum decline with increasing age, levels of dopamine decline, and synapses/receptors are reduced or binding to receptors is reduced. In fact, there occurs a general decrease in D-receptor density with age and a significant age-related decline in dopamine receptors in the anterior cingulate cortex, frontal cortex, lateral temporal cortex, hippocampus, medial temporal cortex, amygdala, medial thalamus and lateral thalamus. The dopamine deficiency with age is responsible for many neurological symptoms that increase in frequency with age, such as decreased arm swing and increased rigidity, and age-related changes in cognitive flexibility.
Serotonin: Serotonergic pathways regulate cognitive processes and behaviors such as arousal (wakefulness), body temperature regulation, emotion and mood, potentially including aggression, feeding and energy homeostasis, sensory perception and have a minor role in reward response. Serotonin influences appetite, sleep, memory and learning, temperature, mood, behavior, muscle contraction, and function of the cardiovascular system and endocrine system.
Decrease in serotonin receptors and the serotonin transporter occur with age [53]. The PET studies in human subjects show that with age the number of theS1 receptors in the caudate nucleus, putamen and frontal cerebral cortex decrease. There is also a decreased binding capacity for serotonin transporter in the thalamus and midbrain [54]. Seroton in seems to have a role in depression and depressed patients have been found to have lower concentrations of the metabolites of serotonin in their cerebrospinal fluid and brain tissue.
C. Norepinephrine and Epinephrine have focus on the central nervous system and regulate sleep patterns and alertness. Epinephrine takes part in controlling the adrenal glands and also plays a role in the fight-or-flight response.
The noradrenergic pathways regulate cognitive processes and behaviours such as anxiety, arousal (wakefulness), circadian rhythm, cognitive control and working memory (co-regulated by dopamine). They have a role in feeding and energy homeostasis, medullary control of respiration, negative emotional memory, nociception, and a minor role in reward-related response.
D. Histamine: Histamine influences sleep, arousal, attention, processing of sensory information in the cerebral cortex, and important aspects of emotion including aggression and mood regulation. It is also involved in learning and memory, and in the regulation of feeding and energy balance. In the CNS, it works with specifically the hypothalamus (tuberomammillary nucleus) and CNS mast cells.
Figure 5: Neurotransmitters and the Stimulatory Effects.

E. Acetylcholine: Acetylcholine, the first neurotransmitter discovered in the peripheral and central nervous systems, activates skeletal muscles in the somatic nervous system and may either excite or inhibit internal organs in the autonomic system (Figure 5). It is the transmitter at the neuromuscular junction connecting motor nerves to muscles. It acts in various regions of the brain through multiple types of receptors, including nicotinic and muscarinic receptors. Cholinergic pathways regulate cognitive processes and behaviors such as arousal (wakefulness), emotion and mood, learning, motor function, motivation, short-term memory and play a minor role in reward response.
F. Glutamate: Glutamate is used at the most fast excitatory synapses in the brain and spinal cord. It is also used at most synapses that are modifiable, i.e. capable of increasing or decreasing in strength. Modifiable synapses are thought to be the main memory-storage elements in the brain. Excessive glutamate release can over stimulate the brain and lead to excitotoxicity response causing cell death resulting in seizures or strokes. This excitotoxicity phenomenon has been implicated in certain chronic diseases including ischemic stroke, epilepsy, amyotrophic lateral sclerosis, AD, Huntington disease, and Parkinson's disease.
Glutamate is another important neurotransmitter that tends to decrease with age [55]. The studies have shown that older subjects have a lower glutamate concentration in the motor cortex compared to younger subjects, and there occurs a significant decline with age, especially in parietal gray matter, basal ganglia and frontal white matter [56]. The parietal and basal ganglia regions are often affected in degenerative brain diseases and show decreased glutamate concentration and activity [57].
G. Other neurotransmitter systems: GABA is used at the majority of fast inhibitory synapses in virtually every part of the brain, and many sedative and tranquilizing drugs act by enhancing the effects of GABA. Correspondingly, glycine is the inhibitory transmitter in the spinal cord at level of Renshaw cells. Aspartate is transmitter at climbing fibers. In addition, there are over 50 neuro active peptides, including p-endorphin and substance-P
Vascular factors
The white matter lesions (WML) have been related to increased cerebro vascular risk and a reduction in cerebral blood flow, cerebral reactivity and vascular density. They may also associated with further tissue changes in grey matter. The WML are found more in frontal rather than posterior brain regions. They seem to impair frontal lobe function regardless of their location [58]. Further, the WML increase with age and show levels of heritability and are common in the elderly even when asymptomatic. Associated with aging and related to blood pressure and vascular factors, other changes include strokes and small vessel disease. The research provides evidence that vascular factors contribute to cognitive dysfunction with aging.
Further, the vascular dementia (VaD) is most frequent in the elderly amounting to about 15%-30%, second only to AD, which is found in about 40%-70% cases [59]. Risk factors to aging and development of VaD include hypertension, diabetes, hyperhomocysteinaemia, and a high cholesterol. The prevalence of dementia increases almost exponentially with increasing age with around 20% of those aged 80 affected rising to 40% of those aged 90 [60].
Hormonal factors
The failing sex hormones in men with andropause and in women at menopause, affect cognitive function with aging. The women have a higher incidence of AD [61]. The research suggests that estrogen therapy in women may increase dopaminergic responsivity [62]. The hormone replacement therapy (HRT) appears to play a protective role [63]. The growth hormone levels also decline with age. It may be associated with falling cognitive performance with age, though the evidence from research is not conclusive [64].
Hypothalamus inflammation and GnRH
A recent study suggests that the inflammation of the hypothalamus may be connected to the overall aging process. The activation of the protein complex NF-kB in mice studies, showed increased activation as mice test subjects aged. This activation not only affects aging, but also affects a hormone, gonadotropin- releasing hormone (GnRH), which has shown certain anti-aging properties when injected into mice outside the hypothalamus, while it causes the opposite effect when injected into the hypothalamus [65].
Subclinical and Clinical Manifestations of Aging Brainn
Neuropsychological changes
Asevidentonthefunctionallevel,thereoccurchangesincognition and memory impairment due to changes in neurotransmitters and various receptors. Genetics, neurotransmitters, hormones and neuroplasticity all play a part in brain aging.
Changes in orientation: The deficit in orientation is an initial and common symptom of brain disease. The recent research suggests that normal aging is usually not associated with significant declines in orientation, a mild deficit, though, may be a part of normal aging [66].
Changes in attention: Many older adults suffer with a decline in attentional abilities. Since the human brain has limited attention span, people use their attention to zone in on specific stimuli and block out others. Studies have found that older adults have a more difficulty encoding and retrieving information when their attention is divided compared to younger adults. The same is true for the tasks of sustained attention. Further, the studies suggest that the sustained attention increases in early adulthood and then remains relatively stable, at least through the seventh decade of life [67]. It may decline afterwards. The attention may also be affected due to other factors like sensory deficits that impact older adults, for example, impaired hearing or vision [68].
Changes in memory: The memory functions, specifically those associated with the medial temporal lobeare susceptible to the age- related decline. The frontal lobes and frontal-striatal dopaminergic pathways are also affected with aging and manifest as memory loss [69]. With the cognitive impairment with aging memory function is commonly affected. But, there is a significant individual variation of effects of aging on frontal lobe neurons and memory deficit [70].
Memory can be episodic memory, semantic memory, procedural memory and working memory. The first two of these are important with regard to aging. Episodic memory is the form of memory in which information is stored with tags like where, when and how. Episodic memory performance declines from middle age onwards affecting the recall in normal aging. Semantic memory is the memory for meanings. It increases gradually from middle age to the young elderly but then declines in the very elderly. These changes occur because the very elderly have slower reaction times, lower attentional levels, slower processing speeds, detriments in sensory and or perceptual functions, or potentially a lesser ability to use strategies compared to younger elderly [71].
The actual level of brain activation, as shown in neuro imaging, may be related more directly to the levels of memory performance [72]. The increased symmetrical hemispheric activation occurring in the frontal lobes is accompanied with changes in memory performance and with the possible white matter changes [73]. But, other factors such as changes in
neurotransmitter or hormone levels are also important. Further, the white matter lesions in frontal lobe impair memory and other cognitive functions [74].
Changes in language: With aging, there occurs decline in the tasks related to word retrieval, comprehension of sentences, synthesis and production of sentences, affecting the totality of the verbal task [75]. The findings from the Nun Study also underlined the linguistic decline with aging [76].
Measures to Retard the Cognitive Aging
The inherent factors
Cognitive reserve: This is the individual ability to demonstrate few signs of cognitive decline with aging despite an aging brain. Studies of cognitive reserve link the specific biological, genetic and environmental factors that make one person susceptible to cognitive decline, and allow another to age more gracefully. In this context, in the Nun Study [76] the researchers concluded that the early idea density was a significant predictor of lower risk for developing AD in old age, whereas the lower idea density was associated with lower brain weight, more brain atrophy, and more neurofibrillary tangles.
Cholesteryl ester transfer protein (CETP): There are certain biomarkers identified as protective against the negative effects of aging. The cholesteryl ester transfer protein (CETP) gene plays an essential role in regulating cholesterol homeostasis, has been linked to prevention of cognitive decline and AD, and held a candidate susceptibility gene for late-onset Alzheimer's disease (LOAD).
It has been documented that the valine CETP homozygotes but not heterozygotes have a relatively 51% less decline in memory compared to a control reference group. Some studies suggested that the CETP I405V polymorphism (rs5882) is associated with a slower rate of memory decline and a lower risk of incident dementia [77]. Whereas, using data from two ongoing epidemiologic clinical- pathologic cohort studies of aging and dementia in the United States, the Religious Order Study and the Memory and Aging Project, another study suggests that the CETP I405V polymorphism was associated with a faster rather than a slower rate of decline in cognition over time, and an increased risk of incident AD.
This finding was collaborated with data showing that the CETP I405V is associated with increased neuritic plaque density at autopsy [78]. Finally, a recent study supports for decreased rate of cognitive decline in carriers of the CETP I405V variant. It documented no CETP-association with the risk for AD or with the rate of decline of AD. The results suggest that the CETP I405V variant influences general cognitive function in a manner that is not directly related to AD pathogenesis [79]. Understanding the mechanism of association between CETP gene and cognitive decline and AD, may open up a future strategy by gene therapy.
The protective factors and measures
Certain factors and measures appears to delay the cognitive decline associated with the aging process. Some of these are specific, such as, high level of education, staying intellectually engaged in mental activities and maintaining social and friendship networks, and underline the importance of regular intellectual exercise. Whereas, others are non-specific and delay the aging process in general, like maintaining a healthy diet, including omega-3 fatty acids, and protective antioxidants.
In addition, a low to moderate alcohol intake may stimulate the areas related to cognitive function, such as prefrontal and hippocampus [80]. It appears to improve cognitive decline [81]. There is some inconclusive evidence that intake of small amounts of alcohol in earlier adult life is protective in later life against cognitive decline and dementia, and a tentative evidence that drinking a small amount of alcohol may decrease the risk of AD later in life [82]. However, a study concluded that findings suggest that, despite previous suggestions, moderate alcohol consumption does not protect older people from cognitive decline [83]. Further, it should be remembered that the elderly are the most sensitive to the toxic effects of alcohol on the brain as pointed out by a French study [84].
A regular physical exercise aiming for fitness in general is a measure to keep healthy, including cognitive health. It increases the executive functioning and reduces the aging-related expected decline of white and grey tissue density. At individual level, the healthy lifestyle that reduces cardiovascular risk, will also benefit the brain. Optimal medical care in this context, offers a protection in terms of cognitive decline with help of anti-hypertensives, antiplatelet, and lipid lowering agents.
Calorie restriction and CRAN
The calorie restriction (CR) is needed to be mentioned as a tool to prevent or slow down aging process and cognitive decline [85]. The concept of calorie restriction with adequate nutrition (CRAN) is a further development in this context [86]. The studies from animal models seem to endorse it [87]. Work on the mechanisms of caloric restriction has given hope to the formulation of future drugs, that is, CRmimetics to increase the human lifespan by simulating the effects of calorie restriction 88].
The CR protects the brain against aging and neurodegeneration through increased activities of plasma membrane redox enzymes (PMRS) like NADH-ascorbate free radical reductase, NADH- quinone oxidoreductase 1, NADH-ferrocyanide reductase, NADH- coenzyme Q10 reductase, and NADH-cytochrome c reductase and antioxidants like a-tocopherol and coenzyme Q10. In a study, the age-related increases in PM lipid peroxidation, protein carbonyls, and nitrotyrosine were attenuated by CR, levels of PMRS enzyme activities were higher, and markers of oxidative stress were lower in cultured neuronal cells treated with CR serum compared with those treated with ad libitum serum [89].
CR has been shown to lower the rate of production of free radicals by mitochondria and to protect cells against oxidative stress [90]. CR appears to attenuate age-related deficits in brain function and protect neurons against dysfunction and apoptosis in animal models of neurodegenerative diseases. The mechanisms by which CR protects neurons may involve induction of the expression of neurotrophic factors, protein chaperones, and mitochondrial uncoupling proteins [91].
The CR has been proven to reduce metabolic rate and oxidative stress, improves insulin sensitivity, and alters neuroendocrine and sympathetic nervous system function. Reduced metabolic rate is another possible explanation for the anti-aging effects of CR, with the consequent reduction in free radicals [92]. There are endocrine changes associated with short-term caloric deprivation (CR or starvation) have been described in rodent models [93]. Many of these alterations were described in studies in human volunteers documenting a drop in triiodothyronine, an increase in cortisol secretion, and a decrease in gonadal function, and the role of leptin, the GH-IGF-I axes, and the thyroid axes in altering markers of aging during prolonged CR [94].
Experimentally, CR has been applied to many species almost universally (with some notable exceptions such as the housefly), resulting in an increase in life span [95]. In the NHP, the Wisconsin study reported an increase in life span and health outcomes in the CR group [96]. The NIH National Institute on Aging 23 years study in rhesus monkeys reported only improved health outcomes and no impact on life span [97]. The CR in Human voluntary studies has been associated with longevity, and factors such as high insulin sensitivity that have been linked with beneficial effects of CR in animal models have also been associated with longevity in humans [98]. Finally, identifying the mechanisms that underlie life prolongation by CR may lead to development of CR mimetics. The health and longevity benefits of CR through CR mimetics such as pharmaceuticals and gene therapies are projected to be applied to wider human population [99].
References
- (2014) Fatality facts in Older people, Insurance Institute for Highway Safety (IIHS). Arlington.
- Denburg NL, Cole CA, Hernandez M, Yamada TH, Tranel D, et al. (2007) The orbitofrontal cortex, real-world decision-making, and normal aging. Ann N Y Acad Sci 1121: 480-498.
- Hess TM, Queen TL (2014) Aging influences on judgment and decision processes: Interactions between ability and experience. In: Verhaeghen P, Hertzog C (Eds.), The Oxford Handbook of Emotion, Social Cognition, and Problem Solving in Adulthood. Oxford University Press, India, pp. 238-255.
- Tucker AM, Stern Y (2011) Cognitive reserve in aging. Curr Alzheimer Res 8(4): 354-360.
- Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, et al. (2014) Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci 69(12): 1536-1544.
- Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18(9): 685-716.
- Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443 (7113): 787-795.
- Trushina E, McMurray CT (2007) Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 145(4): 12331248.
- (2017) What is Neurodegenerative Disease?
- Bredesen DE, Rao RV, Mehlen P (2006) Cell death in the nervous system. Nature 443(7113): 796-802.
- ; Wenk GL (2003) Neuropathologic changes in Alzheimer's disease. J Clin Psychiatry 64 (Suppl 9): 7-10.
- Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19(2): 181-192.
- (2016) Dynamic and metabolic astrocyte-neuron interactions in healthy and diseased brain. In: Christian Giaume, Stephane Oliet (Eds.), Neuroscience 323: 1-222.
- Rodríguez-Arellano JJ, Parpura VR, Zorec R, Verkhratsky A (2016) Astrocytes in physiological aging and Alzheimer's disease. Neuroscience 323: 170-182.
- Hartshorne JK, Germine LT (2015) When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychological Science 26(4): 433-443.
- Craik FIM, Salthouse TA (2000) The Handbook of Aging and Cognition, (2nd edn), Lawrence Erlbaum, New Jersey, USA.
- Raz Naftali, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, et al. (2005) Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15(11): 1676-1689.
- Raz N, Rodrigue KM (2006) Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 30(6): 730748.
- Scahill R, Frost C, Jenkins R, Whitwell JL, Rossor MN, et al. (2003) A longitudinal study of brain volume changes in normal ageing using serial registered magnetic resonance imaging. Arch Neurol 60(7): 989-994.
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, et al. (2003) Mapping cortical change across the human life span. Nat Neurosci 6(3): 309-315.
- Liu T, Lipnicki DM, Zhu W, Tao D, Zhang C, et al. (2012) Cortical Gyrification and Sulcal Spans in Early Stage Alzheimer's Disease. PLoS ONE 7(2): e31083.
- Tao Liu, Wei Wen, Wanlin Zhu, Trollor J, Reppermund S, et al. (2010) The effects of age and sex on cortical sulci in the elderly. NeuroImage 51(1): 19-27.
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, et al. (2004) White matter lesions impair frontal lobe function regardless of their location. Neurology 63(2): 246-253.
- Gunning-Dixon F, Raz N (2003) Neuroanatomical correlates of selected executive functions in middle aged and older adults: a prospective MRI study. Neuropsychologia 41(14): 1929-1941.
- Raz N (2004) The ageing brain: structural changes and their implications for cognitive ageing. In: New frontiers in cognitive ageing. In: Dixon R, Bäckman L, Nilsson L (Eds.), Oxford University Press, Telangana, India, pp. 115-34.
- Anderton BH (2002) Ageing of the brain. Mech Ageing Dev 123(7): 811817.
- Hof PR, Morrison JH (2004) The aging brain: Morpho-molecular senescence of cortical circuits. Trends Neurosci 27(10): 607-613.
- Bryan K, Gibb R, Robinson TE (2003) Brain plasticity and behavior. Current Directions in Psychological Science 12(1): 1-5.
- Finch CE (2003) Review-Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging 24 (Suppl 1): S123-S127.
- Toescu EC, Verkhratsky A, Landfield PW (2004) Review Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci 27(10): 614-620.
- Barnes C, Burke S (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci 7(1): 30-40.
- Mattson MP, Maudsley S, Martin B (2004) BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27(10): 589-594.
- Hof PR, Morrison J (2004) The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci 27(10): 607-613.
- Ng C, Panay N (2004) Hormone replacement therapy update. Geriatric Medicine. 3427-3434.
- Craig M, Cutter W, Wickham H, van Amelsvoort TA, Rymer J, et al. (2004) Effect of long term estrogen therapy on dopaminergic responsivity in post-menopausal women-a preliminary study. Psychoneuroendocrinology 29(10): 1309-1316.
- Herlitz A, Yonker J (2004) Hormonal effects on cognition in adults. In: New frontiers in cognitive ageing. In: Dixon R, Backman L, Nilsson L (Eds.), Oxford University Press, India, pp. 253-278.
- Sytze van Dam P, Aleman A (2004) Insulin - like growth factor-I, cognition and brain ageing. Eur J Pharmacol 490(1-3): 87-95.
- Davis P, Morris J, White DL (1991) The distribution of tangles, plaques, and related immuno-histo-chemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging 12(4): 295-312.
- Whalley LJ, Deary IJ, Appleton CL, Starr JM (2004) Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev 3(4): 369-382.
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, et al. (2005) Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 64(7): 1152-1156.
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, et al. (2006) The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett 406(3): 260-264.
- Kadakkuzha BM, Komolitdin A, Capo RC, Anthony C Carvalloza, Mohammad Fallahi, et al. (2013) Age-associated bidirectional modulation of gene expression in single identified R15 neuron of Aplysia. BMC Genomics 14(1): 880.
- Bernstein H, Payne CM, Bernstein C, Garewal H, Dvorak K (2008) Cancer and aging as consequences of un-repaired DNA damage. New Research on DNA Damage. In: Honoka Kimura and Aoi Suzuki (Eds.), Nova Science Publishers Inc., New York, USA, pp. 1-47.
- Mandavilli BS, Rao KS (1996) Accumulation of DNA damage in aging neurons occurs through a mechanism other than apoptosis. J Neurochem 67(4): 1559-1565.
- Melov S (2004) Review Modeling mitochondrial function in aging neurons. Trends Neurosci 27(10): 601-606.
- Lu T, Pan Y, Kao SY, Li C, Kohane I, et al. (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429(6994): 883-891.
- Mattson M, Maudsley S, Martin B (2004) BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27(10): 589-594.
- Volchegorskii I, Shemyakov S, Turygin V, Malinovskaya NV (2004) The age dynamics of monoamine oxidase activity and levels of lipid peroxidation products in the human brain. Neurosci Behav Physiol 34(4): 303-305.
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, et al. (1998) Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse 30(1): 56-61.
- Ota M, Yasuno F, Ito H, Seki C, et al. (2006) Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-[p-11C]-DOPA. Life Sci 79(8): 730-736.
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, et al. (2000) Age- related dopamine D2/D3 receptor loss in extra-striatal regions of the human brain. Neurobiol Aging 21(5): 683-688.
- Mukherjee J, Christian B, Dunigan K, Shi B, Narayanan TK, et al. (2002) Brain imaging of 18F-Fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to ageing effects on dopamine D-2/D-3 receptors. Synapse 46(3): 170-188.
- Yamamoto M, Suhara T, Okubo Y, Ichimiya T, Sudo Y, et al. (2001) Age- related decline of serotonin transporters in living human brain of healthy males. Life Sci 71(7): 751-757.
- Marcusson J, Oreland L, Winblad B (1984) Effect of age on human brain serotonin (S-1) binding sites. J Neurochem 43(6): 1699-1705.
- Chang L, Jiang CS, Ernst T (2009) Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging 27(1): 142-145.
- Sailasuta N, Ernst T, Chang L (2008) Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn Reson Imaging 26(5): 667-675.
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW (2005) Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging 26(5): 665-672.
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, et al. (2004) White matter lesions impair frontal lobe function regardless of their location. Neurology 63(2): 246-253.
- Fratiglioni L, Launer L, Anderson K, Breteler MM, Copeland JR, et al. (2000) Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54(11 Suppl 5): S10-S15.
- Lobo A, Launer L, Fratiglioni L, Andersen K, Di Carlo A, et al. (2000) For the Neurologic Diseases in the Elderly Research Group. Prevalence of dementia and major subtypes in Europe: a collaborative study of population based-cohorts. Neurology 54(11 Suppl 5): S4-S9.
- Ng C, Panay N (2004) Hormone replacement therapy update. Geriatric Medicine 34: 27-34.
- Craig M, Cutter W, Wickham H, van Amelsvoort TA, Rymer J, et al. (2004) Effect of long term estrogen therapy on dopaminergic responsivity in postmenopausal women-a preliminary study. Psychoneuroendocrinology 29(10): 1309-1316.
- Herlitz A, Yonker J (2004) Hormonal effects on cognition in adults. New frontiers in cognitive ageing. In: Dixon R, Backman L, Nilsson L (Eds.), Oxford University Press, India, pp. 253-278.
- Sytze van Dam P, Aleman A (2004) Insulin-like growth factor-I, cognition and brain ageing. Eur J Pharmacol 490(1-3): 87-95.
- Guo J, Juxue L, Purkayastha P, Yizhe Tang, Hai Zhang, et al. (2013) Hypothalamic programming of systemic ageing involving IKK-[bgr], NF- [kgr]B and GnRH. Nature 497: 211-216.
- Kensinger EA, Suzanne Corkin (2009) Cognition in aging and age related disease. In: Hof PR, Mobbs CV (Eds.), Handbook of the neuroscience of aging. Elsevier Press, London, pp. 249-256.
- Banich MT, Compton RJ (2011) Cognitive neuroscience. Belmont, Wadsworth.
- Carrier JSA, Cheyne A, Solman GJF, Smilek D (2010) Age trends for failures of sustained attention. Psychol Aging 25(3): 569-574.
- Light LL (1991) Memory and aging: Four hypotheses in search of data. Annual Review of Psychology 42: 333-376.
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, et al. (2002) Variable effects of aging on frontal lobe contributions to memory. Neuroreport 13(18): 2425-2428.
- Cabeza R (2004) Neuroscience frontiers of cognitive ageing: approaches to cognitive neuroscience of ageing. New frontiers in cognitive ageing. In: Dixon R, Backman L, Nilsson L (Eds.), Oxford University Press, India, pp. 179-198.
- Rosen A, Prull M, O'Hara R, Race EA, Desmond JE, et al. (2002) Variable effects of ageing on frontal lobe contributions to memory. Neuroreport 13(18): 2425-2428.
- Cabeza R, Daselaar S, Dolcos F, Prince SE, Budde M, et al. (2004) Task- independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex 14(4): 364-375.
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, et al. (2004) White matter lesions impair frontal lobe function regardless of their location. Neurology 63(2): 371-381.
- Crosson B, Garcia A, Mcgregor K, Wierenga CE (2013) The Impact of Aging on Neural Systems for Language. In: MFG Koffler S, Morgan J, Baron IS (Eds.), Neuropsychology 1: 149-187.
- Riley KP, Snowdon DA, Desrosiers MF, Markesbery WR (2005) Early life linguistic ability, late life cognitive function, and neuropathology: Findings from the Nun Study. Neurobiol Aging 26(3): 341-347.
- Yu L, Shulman JM, Chibnik L, Leurgans S, Schneider JA, et al. (2011) The CETP I405V polymorphism is associated with an increased risk of Alzheimer's disease. Aging Cell 11(2): 228-233.
- Li Q, Huang P, ChaoHe Q, Lin QZ, Wu J, et al. (2014) Association between the CETP polymorphisms and the risk of Alzheimer's disease, carotid atherosclerosis, longevity, and the efficacy of statin therapy. Neurobiol Aging 35(6): 1513.e13-e23.
- Munger C, Perkes A, Peterson M, Schmutz C, Leary M, et al. (2015) Population-based analysis of CETP identifies association between I405V and cognitive decline: The Cache County Study. Neurobiol Aging 36(1): 547.e1-547.e3.
- Stampfer MJ, Kang JH, Chen J, Rebecca Cherry, Francine Grodstein, et al. (2005) Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med 352: 245-253.
- Arntzen, Schirmer H, Wilsgaard T, Mathiesen EB (2010) Moderate wine consumption is associated with better cognitive test results: A 7 year follow up of 5033 subjects in the Troms0 study. Acta Neurol Scand Suppl (190): 23-29.
- Peters R, Peters J, Warner J, Beckett N, Bulpitt C (2008) Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing 37(5): 505-512.
- Cooper Claudia, Bebbington Paul, Meltzer Howard, Jenkins Rachel, Brugha Traolach, et al. (2009) Alcohol in moderation, premorbid intelligence and cognition In Older Adults: results from the Psychiatric Morbidity Survey. J Neurol Neurosurg Psychiatry 80(11): 1236-1239.
- Pierucci-Lagha A, Derouesne C (2003) Alcoholism and aging. Alcoholic dementia or alcoholic cognitive impairment? Psychol Neuropsychiatr Vieil 1(4): 237-249.
- Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R (2010) Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci 65(7): 695-703.
- Spindler SR (2010) Biological Effects of Calorie Restriction: Implications for Modification of Human Aging. The Future of Aging, pp. 367-438.
- Anderson RM, Shanmuganayagam D, Weindruch R (2009) Caloric Restriction and Aging: Studies in Mice and Monkeys. Toxicol Pathol 37(1): 47-51.
- Contestabile A (2009) Benefits of caloric restriction on brain aging and related pathological States: understanding mechanisms to devise novel therapies. Curr med chem 16(3): 350-361.
- Hyun DH, Emerson SS, Jo DG, Mark P Mattson, Rafael de Cabo (2006) Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. PNAS 103(52): 19908-19912.
- Agarwal S, Siddharth Sharma, Vineeth Agarwal, Nilanjan Roy (2005) Caloric restriction augments ROS defense in S. cerevisiae, by a Sir2p independent mechanism. Free Radic Res 39(1): 55-62.
- Merry BJ (2004) Oxidative stress and mitochondrial function with aging- the effects of calorie restriction. Aging Cell 3(1): 7-12.
- Heilbronn LK, Ravussin E (2003) Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 78(3): 361-369.
- Masoro EJ (1988) Food restriction in rodents: an evaluation of its role in the study of aging. J Gerontol 43(3): B59-B64.
- Shimokawa I, Higami Y (2001) Leptin and anti-aging action of caloric restriction. J Nutr Health Aging 5(1): 43-48.
- Roberts SB, John Speakman J (2013) Update on human calorie restriction research. Adv Nutr 4: 563-564.
- Ramseya JJ, Colmana RJ, Binkleya NC, Christensen JD, Gresl TA, et al. (2000) Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol 35(9-10): 1131-1349.
- Ingram DK, Mattison JA, de Cabo R, Roth GS (2015) History of the Study of Calorie Restriction in Nonhuman Primates Conducted by the National Institute on Aging: The First Decade. In: Yu B (Ed.), Nutrition, Exercise and Epigenetics: Ageing Interventions. Healthy Ageing and Longevity, pp 245-275.
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, et al. (2006) One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 61(9): 943-950.
- McDonald RB, Ramsey JJ (2010) Honoring Clive McCay and 75 Years of Calorie Restriction Research. J Nutr 140(7): 1205-1210.
© 2017 Vinod Nikhra. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






